January 11, 2024 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A three-step mechanism explaining ultraviolet-induced CO desorption from CO ice
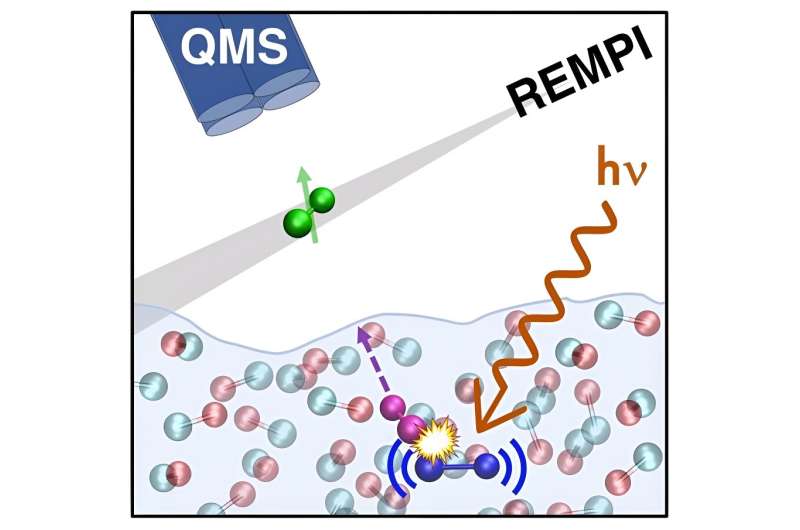
The desorption of CO ice induced by ultraviolet (UV) radiation is a phenomenon that occurs in some cold parts of the universe, which has often also been replicated in laboratory settings. While this phenomenon is now well-documented, the molecular mechanisms underpinning it are yet to be fully uncovered.
Researchers at University of Lille and Sorbonne University, in the framework of the French ANR PIXyES project led by Mathieu Bertin, recently carried out a study investigating this mechanism through a combination of experiments and molecular simulations. Their paper, published in Physical Review Letters, outlines a three-step mechanism that could give rise to UV photon-induced CO ice desorption.
"In the interstellar medium (ISM), molecular matter is mostly found in the coldest and densest regions," Maurice Monnerville and Jean-Hugues Fillion, the leading authors of the paper, told Phys.org.
"These areas are stellar nurseries, where stars and planets come to life, like the inner parts of protoplanetary disks and pre-stellar clouds. About 200 different molecular species, ranging from simple ones, like hydrogen and water (H2, H2O, CO,…) to more complex ones like methanol (CH3OH) coexist with tiny grains made of silicates and carbons."
In some of the coldest regions of the universe with extremely low temperatures of approximately 10 K, all molecules (except H2) stick on the surface of tiny grains, forming icy layers. These layers are primarily made up of condensed water and various other substances, such as carbon monoxide (CO) and carbon dioxide (CO2).
"These interstellar ices act as a crucial reservoir of molecular matter in the cold regions of the universe," the authors explained.
"In these coldest regions, abnormal abundances in the gaseous phase have been detected, even though the species should be frozen on dust grains due to the extremely low temperature. So, how can the desorption of these molecules in the cold regions of space be explained? To understand these unexpected abundances, a non-thermal desorption phenomenon explaining the detection of these molecules in the gaseous phase is necessary."
One process that can explain the high abundance of gaseous molecules in parts of the universe with particularly low temperatures is the desorption induced by UV photons from surrounding stellar emission, filtered through atomic hydrogen (7–13.6 eV). Many physicists have thus been recently exploring this phenomenon, particularly the UV photo-desorption of CO, in great depth.
"CO ices serve as a potential starting point for complex chemistry leading to the formation of methanol and subsequent highly diverse organic chemistry," the authors said. "For these reasons, the VUV-photodesorption of solid CO has been for decades the subject of a large panel of experimental studies aiming to provide absolute desorption yields to the astrochemical community."
Previous research efforts by the research group of Jean-Hugues Fillion in the LERMA lab at Sorbonne University found evidence suggesting that the UV-induced desorption mechanism of CO is in great part indirect. This essentially means that the desorbing molecule is not necessarily the one absorbing the photon, but rather that this desorption process is primarily driven by a transfer of energy between the excited and the surface molecule.
So far, however, this desorption mechanism remained poorly understood, as neither theoretical nor experimental works were able to account for all its associated molecular properties. The primary objective of the recent study by Monnerville and his colleagues was thus to characterize the mechanism, with a keen focus on the nature of the energy transfer they previously reported and the properties of desorbed molecules.
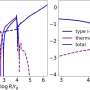
"We have developed a concerted strategy between theory and experiments," Monnerville said. "The PCMT team at Lille University used Ab Initio Molecular Dynamics (AIMD), a sophisticated mixed quantum/classical simulation technique based on density functional theory (DFT) to further elucidate the energy transfer mechanism."
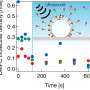
"Concurrently, the Parisian team performed new pulsed laser-induced photodesorption at selected excitation energy in the VUV using the SPICES ultrahigh vacuum setup providing data on the vibrational and translational energy distribution of the photodesorbed CO molecules which can be directly compared to the AIMD outcomes."
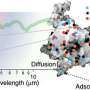
The simulations performed by the part of this research group based at Lille University revealed that the UV radiation-induced desorption of CO ice relied on a mechanism with three key stages. During the first of these stages, an excited molecule vibrates within the ices, retaining the vibrational energy initially deposited within it.
"Subsequently, the excited molecule and one or two CO molecules in its vicinity begin to be mutually attracted and consequently gain translational energy, leading to their collision via a 'kick event,'" Monnerville explained.
"The colliding molecules then initiate movement and interact with other molecules within the ice, resulting in a cascade energy transfer effect. Essentially, the translational and rotational energy acquired in the second step is transferred to surface CO molecules, enabling them to overcome the binding energy of the aggregate and desorb."
Notably, the three key steps outlined by Monnerville and his collaborators are partly aligned with the well-known DIET (Desorption Induced by Electronic Transition) mechanism. This mechanism was previously hypothesized to be as a possible cause of VUV irradiation-induced desorption of interstellar ice analogs, yet this study is the first to describe it in detail via simulations that also agree with experimental observations.
"The key to successfully describing this complex process and achieving perfect agreement with experimental observations lay in the use of a computationally intensive simulation techniques, which allowed for a more precise depiction of this complex dynamical system," Monnerville said.
"The AIMD techniques were crucial for accurately characterizing the interaction between a vibrationally excited CO molecule and its neighbors, initiating the desorption process—a facet where previous theoretical studies had fallen short."
The recent work by this team of researchers is a significant contribution to the study of molecular processes in ultracold environments. Remarkably, it is the first to provide detailed simulations of the mechanism behind UV-induced CO ice desorption that are perfectly aligned with experimental observations.
"Our discovery of a three-step mechanism (vibrational excitation, kick, desorption) enables us to explain a complex process in relatively simple terms," the authors said. "It is in fact the simplicity of this mechanism that makes it significant. It is quite plausible that this primary mechanism could be used by the astrophysical community to theoretically explain the desorption already observed in more complex interstellar ices."
In the future, the experimental methods and simulation tools employed by the Lille and Sorbonne University teams could be used to study the photo-desorption of a wider range of complex ice mixture. The researchers are now also working on a machine learning-based potential energy surface (PES) model trained using data gathered during their ab initio molecular dynamics (DFT) calculations.
"This High-Dimensional Neural Network PES will enable us to perform more and longer molecular dynamics simulations of the CO desorption process on a more representative model of the CO ice surface, while significantly reducing computational costs," Monnerville added.
"Additionally, we are conducting new experimental and theoretical studies on more complex interstellar ice analogs, such as CO2, CO:N2, and CO:NO, using similar methodologies. Finally, a novel experimental approach will be tested to reveal the angular distribution of photodesorbed molecules. This will be achieved by implementing a velocity map imaging detector, a powerful detection technique well-known for gas phase applications, though its development is challenging for the study of desorbed molecules from cold substrates."
More information: Samuel Del Fré et al, Mechanism of Ultraviolet-Induced CO Desorption from CO Ice: Role of Vibrational Relaxation Highlighted, Physical Review Letters (2023). DOI: 10.1103/PhysRevLett.131.238001
Journal information: Physical Review Letters
© 2024 Science X Network