This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Using generative AI to identify potent and selective MYT1 inhibitors for the treatment of cancer
Recent research has identified MYT1 as a promising new therapeutic target for breast and gynecological cancer and discovered a series of novel, potent, and highly selective inhibitors specifically targeting MYT1.
These findings, published in the Journal of Medicinal Chemistry, were supported by Insilico Medicine's AI-driven generative biology and chemistry engine.
Across the world, breast and gynecological cancers pose serious threats to women's health, fertility, and overall quality of life. In order to identify potential targets for new therapeutics, the research team leveraged Insilico's proprietary AI-driven target identification platform, PandaOmics, to analyze data on five forms of gynecological cancers, including ovarian, endometrial, cervical, and breast cancer, particularly triple-negative breast cancer.
Remarkably, MYT1 consistently ranked at the forefront across all diseases regarding relevance.
MYT1 is a member of the Wee1-kinase family, rarely expressed in most normal tissues but highly expressed in most cancer types. It has been reported that MYT1 inhibition and CCNE1 amplification, a condition known as synthetic lethality, play crucial functions in cell cycle regulation, which indicates MYT1 inhibition is a promising synthetic lethal therapeutic strategy for the treatment of cancers with genome instability (e.g. CCNE1 amplification).
However, MYT1 is highly homologous to Wee1, which makes it challenging to design selective MYT1 inhibitors. In this study, Insilico addressed the gap in selective MYT1 inhibitors with the support of Chemsitry42, Insilico's AI-driven small molecule generation platform.
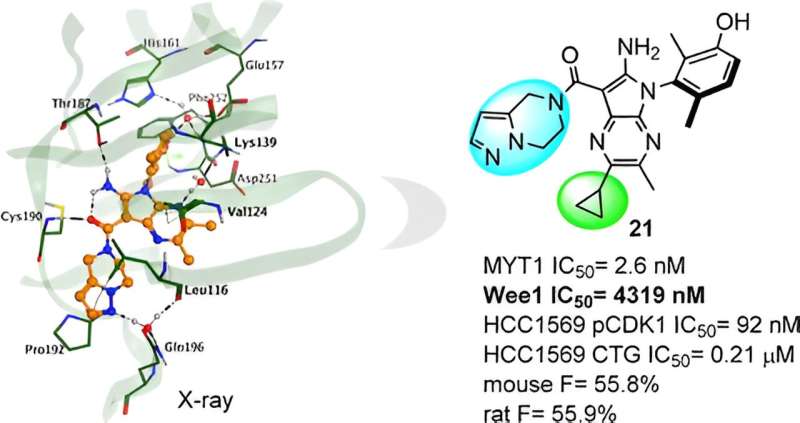
Using structure-based drug design (SBDD) strategies and applying rigorous filters for similarity and selectivity, Insilico designed an array of compounds targeting MYT1 from scratch. Among these novel compounds, one series emerged as hit compounds.
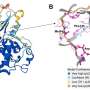
Insilico then conducted an X-ray crystal structure analysis of the complex and found a significant impact on the activity of subtle chemical structure modifications. This knowledge provided guidance for further molecular optimization, leading Insilico to the discovery of the lead compound, Compound 21.
Compound 21 presents good MYT1 activity, and excellent selectivity over Wee1, and the other kinase panel reduces the potential risk for off-target effects and might translate to a safer profile. In preclinical studies, it also shows potent in vivo antitumor efficacy and a promising profile in ADME and PK/PD.
"The innovative approach of this program has not only presented a method for effective target identification but has also led to the development of a promising selective MYT1 inhibitor," said Yazhou Wang, Ph.D., medicinal chemistry leader of the MYT1 program from Insilico Medicine, and the first author of this paper. "Compound 21 expands Insilico's synthetic lethal pipeline and paves the way toward a safer, more effective therapeutic future for patients battling gynecological and breast cancers."
More information: Yazhou Wang et al, Discovery of Tetrahydropyrazolopyrazine Derivatives as Potent and Selective MYT1 Inhibitors for the Treatment of Cancer, Journal of Medicinal Chemistry (2023). DOI: 10.1021/acs.jmedchem.3c01476
Journal information: Journal of Medicinal Chemistry
Provided by InSilico Medicine