This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
How to make bright quantum dots even brighter
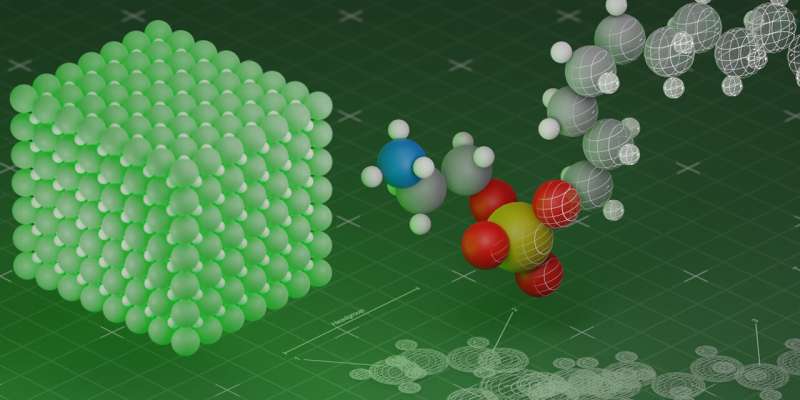
Quantum dots are a kind of artificial atom: just a few nanometers in size and made of semiconductor materials, they can emit light of a specific color or even single photons, which is important for quantum technologies. The discoverers and pioneers of the commercial production of quantum dots were awarded the Nobel Prize in Chemistry in 2023.
In recent years, quantum dots made of perovskites have attracted particular attention. Perovskites belong to a class of materials that have a similar structure to the mineral perovskite (calcium titanate). Quantum dots made of such materials were produced for the first time by ETH Zurich in 2015.
These quantum dots made of perovskite nanocrystals can be mixed with liquids to form a dispersion, which makes them easy to process further. Moreover, their special optical properties make them shine more brightly than many other quantum dots. They can also be produced more cheaply, which makes them interesting for applications in displays, for instance.
A team of researchers led by Maksym Kovalenko at ETH Zurich and Empa, working in collaboration with counterparts in Ukraine and the U.S., have now demonstrated how these promising properties of perovskite quantum dots can be improved further. They used chemical methods for surface treatment and quantum mechanical effects that had never before been observed in perovskite quantum dots. The researchers recently published their results in two papers in Nature.
Unhappy atoms reduce brightness
Brightness is an important measure for quantum dots and is related to the number of photons the quantum dot emits per second. Quantum dots radiate photons of a specific color (and hence frequency) after being excited, for example, by ultraviolet light of a higher frequency.
This leads to the formation of an exciton consisting of an electron, which can now move more freely, and a hole—in other words, a missing electron—in the energetic band structure of the material. The excited electron can fall back to a lower energy state and thus recombine with the hole. If the energy released during this process is converted into a photon, the quantum dot emits light.
This doesn't always work, however. "At the surface of the perovskite nanocrystals are 'unhappy' atoms that are missing a neighbor in the crystal lattice," senior researcher Gabriele Raino explains. These edge atoms disturb the balance between positive and negative charge carriers inside the nanocrystal and can cause the energy released during a recombination to be converted into lattice vibrations instead of being emitted as light. As a result, the quantum dot "blinks," meaning that it doesn't shine continuously.
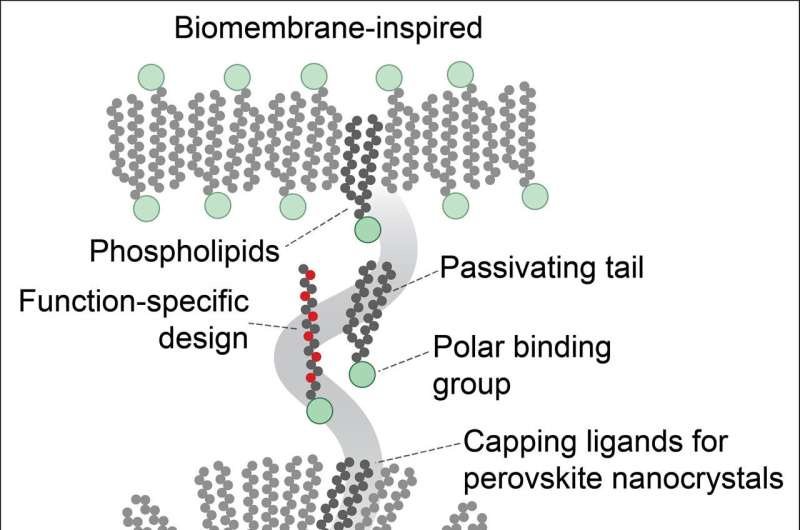
Protective coating made of phospholipids
To prevent this from happening, Kovalenko and his team have developed tailor-made molecules known as phospholipids. "These phospholipids are very similar to the liposomes in which, for instance, the mRNA vaccine against the coronavirus is embedded in such a way as to make it stable in the bloodstream until it reaches the cells," Kovalenko explains.
An important difference: the researchers optimized their molecules so that the polar (electrically sensitive) part of the molecule latches onto the surface of the perovskite quantum dots and makes sure that the "unhappy" atoms are provided with a charge partner.
The nonpolar part of the phospholipid that protrudes on the outside also makes it possible to turn quantum dots into a dispersion inside non-aqueous solutions such as organic solvents. The lipid coating on the surface of the perovskite nanocrystals is also important for their structural stability, as Kovalenko emphasizes: "This surface treatment is absolutely essentially for anything we might want to do with the quantum dots."
So far, Kovalenko and his team have demonstrated the treatment for quantum dots made of lead halide perovskites, but it can also be easily adapted to other metal halide quantum dots.
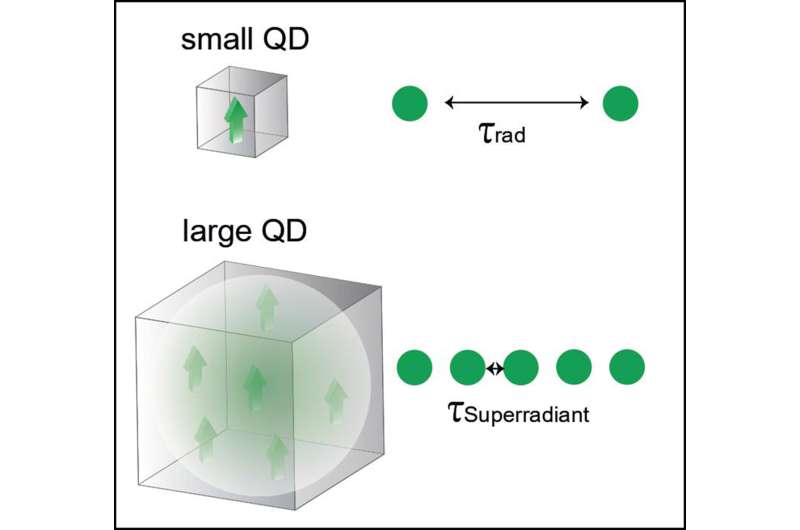
Even brighter thanks to superradiance
With the lipid surface it was possible to reduce the blinking of the quantum dots to such an extent to emit a photon in 95% of electron-hole recombination events. To make the quantum dot even brighter, however, the researchers had to increase the speed of the recombination itself—and that requires quantum mechanics.
An excited state, such as an exciton, decays when a dipole—positive and negative charges displace with respect to each other—interacts with the electromagnetic field of the vacuum. The larger the dipole, the faster the decay. One possibility of creating a larger dipole involves coherently coupling several smaller dipoles to each other. This can be compared to pendulum clocks that are mechanically connected and tick in step with each other after a certain length of time.
The researchers were able to show experimentally that the coherent coupling also works in perovskite quantum dots—with only a single exciton dipole that—through quantum mechanical effects—spreads out all over the volume of the quantum dot, thereby creating several copies of itself, as it were. The larger the quantum dot, the more copies can be created. These copies can bring about an effect known as superradiance, by which the exciton recombines much faster.
The quantum dot is consequently also ready more quickly to take up a new exciton and can thus emit more photons per second, making it even brighter. An important detail to note is that the faster quantum dot continues to emit single photons (not several photons at once), which makes it suitable for quantum technologies.
The improved perovskite quantum dots are not only of interest for light production and displays, says Kovalenko, but also in other less obvious fields. For instance, they could be used as light-activated catalysts in organic chemistry. Kovalenko is conducting research into such applications and several others, including within the framework of NCCR Catalysis.
More information: Chenglian Zhu et al, Single-photon superradiance in individual caesium lead halide quantum dots, Nature (2024). DOI: 10.1038/s41586-023-07001-8. www.nature.com/articles/s41586-023-07001-8
Viktoriia Morad et al, Designer Phospholipid Capping Ligands for Soft Metal Halide Nanocrystals, Nature (2023). DOI: 10.1038/s41586-023-06932-6
Journal information: Nature
Provided by ETH Zurich