This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Nanoparticle-delivered RNA reduces neuroinflammation in lab tests
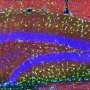
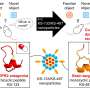
Some COVID-19 vaccines safely and effectively used lipid nanoparticles (LNPs) to deliver messenger RNA to cells. A new MIT study shows that different nanoparticles could be used for a potential Alzheimer's disease (AD) therapy. In tests in multiple mouse models and with cultured human cells, a newly tailored LNP formulation effectively delivered small interfering RNA (siRNA) to the brain's microglia immune cells to suppress the expression of a protein linked to excessive inflammation in Alzheimer's disease.
In a prior study, the researchers showed that blocking the consequences of PU.1 protein activity helps to reduce Alzheimer's disease-related neuroinflammation and pathology. The new results, reported in the journal Advanced Materials, achieve a reduction in inflammation by directly tamping down expression of the Spi1 gene that encodes PU.1.
More generally, the new study also demonstrates a new way to deliver RNA to microglia, which have been difficult to target so far.
Study co-senior author Li-Huei Tsai, Picower Professor of Neuroscience and Director of The Picower Institute for Learning and Memory and Aging Brain Initiative, said she hypothesized that LNPs might work as a way to bring siRNA into microglia because the cells, which clear waste in the brain, have a strong proclivity to uptake lipid molecules.
She discussed this with Robert Langer, David Koch Institute Professor, who is widely known for his seminal work on nanoparticle drug delivery; they decided to test the idea of reducing PU.1 expression with an LNP-delivered siRNA.
"I still remember the day when I asked to meet with Bob to discuss the idea of testing LNPs as a payload to target inflammatory microglia," said Tsai, a faculty member in the Department of Brain and Cognitive Sciences. "I am very grateful to The JPB Foundation, who supported this idea without any preliminary evidence."
Langer Lab graduate student Jason Andresen and former Tsai Lab postdoc William Ralvenius led the work and are the study's co-lead authors. Owen Fenton, a former Langer Lab postdoc who is now an assistant professor at the University of North Carolina's Eshelman School of Pharmacy, is a co-corresponding author along with Tsai and Langer. Langer is a Professor in Chemical Engineering, Biological Engineering, and the Koch Institute for Integrative Cancer Research.
Perfecting a particle
The simplest way to test whether siRNA could therapeutically suppress PU.1 expression would have been to make use of an already available delivery device, but one of the first discoveries in the study is that none of eight commercially available reagents could safely and effectively transfect cultured human microglia-like cells in the lab.
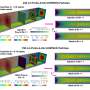
Instead, the team had to optimize an LNP to do the job. LNPs have four main components, and by changing the structures of two of them and varying the ratio of lipids to RNA, the researchers were able to come up with seven formulations to try. Importantly, their testing included trying their formulations on cultured microglia that they had induced into an inflammatory state. That state, after all, is the one in which the proposed treatment is needed.
Among the seven candidates, one the team named "MG-LNP" stood out for its especially high delivery efficiency and safety of a test RNA cargo.
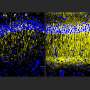
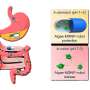
What works in a dish sometimes doesn't work in a living organism, so the team next tested their LNP formulations' effectiveness and safety in mice. Testing two different methods of injection, into the body or into the cerebrospinal fluid (CSF), they found that injection into the CSF ensured much greater efficacy in targeting microglia without affecting cells in other organs.
Among the seven formulations, MG-LNP again proved the most effective at transfecting microglia. Langer said he believes this could potentially open new ways of treating certain brain diseases with nanoparticles someday.
A targeted therapy
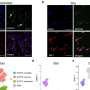
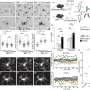
Once they knew MG-LNP could deliver a test cargo to microglia both in human cell cultures and mice, the scientists then tested whether using it to deliver a PU.1-suppressing siRNA could reduce inflammation in microglia. In the cell cultures, a relatively low dose achieved a 42 percent reduction of PU.1 expression (which is good because microglia need at least some PU.1 to live).
Indeed MG-LNP transfection did not cause the cells any harm. It also significantly reduced the transcription of the genes that PU.1 expression increases in microglia, indicating that it can reduce multiple inflammatory markers.
In all these measures and others, MG-LNP outperformed a commercially available reagent called RNAiMAX that the scientists tested in parallel.
"These findings support the use of MG-LNP-mediated anti-PU.1 siRNA delivery as a potential therapy for neuroinflammatory diseases," the researchers wrote.
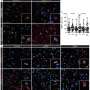
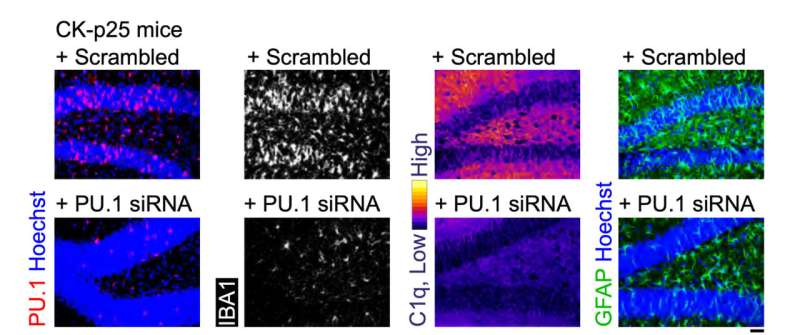
The final set of tests evaluated MG-LNP's performance, delivering the siRNA in two mouse models of inflammation in the brain. In one, mice were exposed to LPS, a molecule that simulates infection and stimulates a systemic inflammation response. In the other model, mice exhibit severe neurodegeneration and inflammation when an enzyme called CDK5 becomes hyperactivated by a protein called p25.
In both models, injection of MG-LNPs carrying the anti-PU.1 siRNA reduced the expression of PU.1 and inflammatory markers, much like in cultured human cells.
"MG-LNP delivery of anti-PU.1 siRNA can potentially be used as an anti-inflammatory therapeutic in mice with systemic inflammation and in the CK-p25 mouse model of AD-like neuroinflammation," the scientists concluded, calling the results a "proof-of-principle." More testing will be required before the idea can be tried in human patients.
More information: William T. Ralvenius et al, Nanoparticle‐Mediated Delivery of Anti‐PU.1 siRNA via Localized Intracisternal Administration Reduces Neuroinflammation, Advanced Materials (2023). DOI: 10.1002/adma.202309225
Journal information: Advanced Materials
Provided by Massachusetts Institute of Technology