This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Elucidating the mechanism of autophagosomes shaping with a flexible web
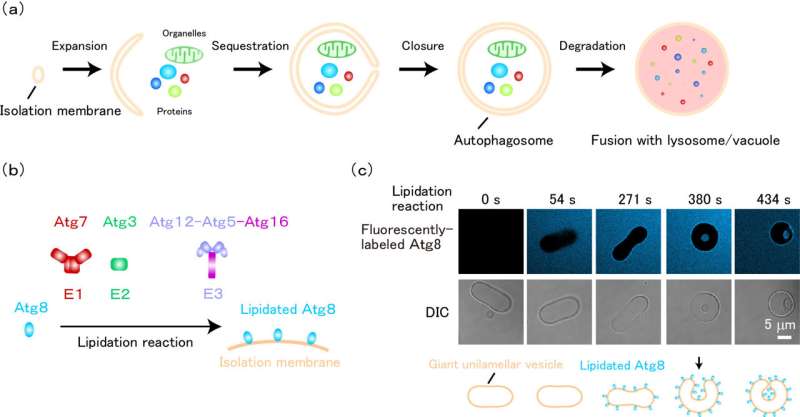
A research group led by Professor Nobuo Noda of Hokkaido University has reconstituted the membrane invagination process required to form autophagosomes in vitro for the first time, showing that this process is mediated by Atg8 protein that plays a central role in autophagy, and a group of enzymes that carry out its lipidation reaction.
Autophagy is a mechanism that breaks down and reuses harmful or unwanted substances in cells. It was previously known that Atg8 and the E1, E2, and E3 enzymes responsible for its lipidation play a central role in autophagy, but their role in autophagosome formation, especially in the process of forming its shape, was poorly understood.
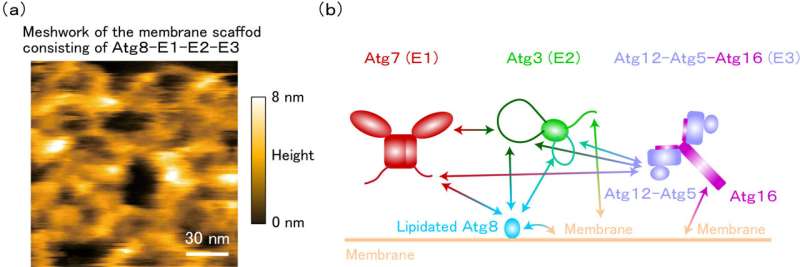
In in vitro experiments, the group found that membranes invaginate when all lipidated Atg8 and E1-E2-E3 enzymes are present. At this time, high-speed atomic force microscopy and nuclear magnetic resonance analysis revealed that these proteins form flexible, higher-order complexes on membranes through intrinsically disordered regions.
Furthermore, yeast experiments confirmed that the E1 enzyme, whose intracellular localization was previously unknown, also localized to the membrane during autophagosome formation along with Atg8 and the E2 and E3 enzymes. These results show that these proteins work together to form autophagosomes.
This study sheds light on the new mechanism of autophagosome formation and is expected to lead to the development of drugs that specifically regulate autophagy. The findings are published in the journal Nature Structural & Molecular Biology.
The research team included Dr. Noda, Senior Researcher, Tatsuro Maruyama and Postdoctoral Researcher, Mohammed Jahangir Alam of the Institute of Microbial Chemistry in collaboration with the group of Dr. Hitoshi Nakatogawa (Professor, Tokyo Institute of Technology).
This result was obtained from JST Strategic Basic Research Programs team-based research area CREST: "Spatiotemporal dynamics of intracellular components." The research topic is "Autophagy dynamics driven by multi-level higher-order structural components."
In this area, researchers aim at an integrated understanding of cells by observing and measuring the dynamics of higher-order structures in cells, from supramolecular complexes to organelles and membrane-free organelles, and analyzing their functional correlations, say the authors.
More information: Jahangir Md. Alam et al, Complete set of the Atg8–E1–E2–E3 conjugation machinery forms an interaction web that mediates membrane shaping, Nature Structural & Molecular Biology (2023). DOI: 10.1038/s41594-023-01132-2
Journal information: Nature Structural & Molecular Biology
Provided by Japan Science and Technology Agency