This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Breakthrough in nitrile activation is promising pathway for anticancer precursor synthesis
A research team, affiliated with UNIST has unveiled a novel method to produce a selective anticancer precursor substance that targets and eliminates cancer cells. This groundbreaking method, previously existing only in theory, has now been experimentally proven for the first time, opening up new possibilities in the development of innovative drugs through extensive research on the effects of anticancer precursors on the human body.
Led by Professor Jaeheung Cho of the Department of Chemistry at UNIST, the research team has successfully demonstrated that the synthesis of hydroxymato cobalt (III), a potential candidate substance for anticancer precursors, involves the reaction of metal-active oxygen species with nitrile. Unlike previous studies that relied on expensive heavy metals, this new method utilizes cost-effective metals and operates at lower temperatures.
The work is published in the journal JACS Au.
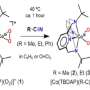
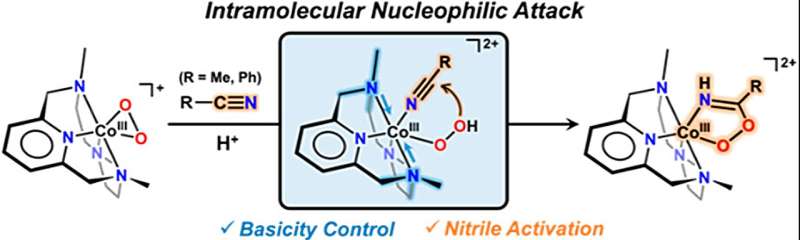
Nitrile, a compound widely used in pharmaceuticals and agricultural pesticides, has proven challenging to synthesize. However, the research team has now confirmed that the reaction between nitriles and cobalt-hydroperoxo species, a type of metal-active oxygen species, leads to the synthesis of peroxyimidateto cobalt (III). This finding reveals that peroxyimidateto cobalt (III) is an intermediate substance formed during the chemical reaction, ultimately producing hydroxymiteto cobalt (III).
To synthesize cobalt (III)-peroxyimidato complexes, the research team introduced a new species known as acobalt(III)-hydroperoxo specifications. Remarkably, they discovered that the reaction occurs when -hydroperoxo is nucleophilic-attacked with nitrile. Moreover, it was observed that the addition of a base to peroxymidato cobalt (III) transforms it into hydroxymito cobalt (III), enabling the synthesis of precursors.
The research team placed particular emphasis on the significance of the basicity of metal-dioxygen specifications, specifically the metal-(hydro)peroxo [M–O2(H)] complex species. By controlling the atoms bound to the cobalt-hydroperoxo species that did not react with nitrile, they successfully increased basicity, thereby enabling rapid reactions even at low temperatures.
To further investigate the structural aspects of cobalt(III)-hydroperoxo specifications, the research team employed computational chemistry simulations, which leverage the power of computer computing to analyze chemical phenomena. These simulations highlighted the impact of changes in the combination of atoms on the structure of cobalt(III)-hydroperoxo specifications, reaffirming the crucial role of basicity.
Professor Cho stated, "This research unveils the underlying mechanisms of metal-active oxygen species in activating nitrile, serving as a foundation for the future development of catalysts capable of activating nitrile."
More information: Yeongjin Son et al, Mechanistic Insights into Nitrile Activation by Cobalt(III)–Hydroperoxo Intermediates: The Influence of Ligand Basicity, JACS Au (2023). DOI: 10.1021/jacsau.3c00532