This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Using tiny traps to study protein interactions can provide new knowledge about difficult-to-treat diseases
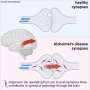
Proteins that form clumps occur in many difficult-to-treat diseases, such as ALS, Alzheimer's, and Parkinson's. The mechanisms behind how the proteins interact with each other are difficult to study, but now researchers at Chalmers University of Technology, Sweden, have discovered a new method for capturing many proteins in nano-sized traps. Inside the traps, the proteins can be studied in a way that has not been possible before.
"We believe that our method has great potential to increase the understanding of early and dangerous processes in a number of different diseases and eventually lead to knowledge about how drugs can counteract them," says Andreas Dahlin, professor at Chalmers, who led the research project.
The research has been presented in the scientific article "Stable trapping of multiple proteins at physiological conditions using nanoscale chambers with macromolecular gates," recently published in Nature Communications.
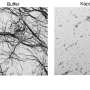
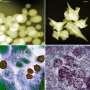
Proteins that form clumps in our bodies cause a large number of diseases, including ALS, Alzheimer's and Parkinson's. A better understanding of how the clumps form could lead to effective ways to dissolve them at an early stage, or even prevent them from forming altogether. Today, there are various techniques for studying the later stages of the process, when the clumps have become large and formed long chains, but until now it has been difficult to follow the early development, when they are still very small. These new traps can now help to solve this problem.
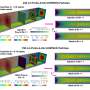
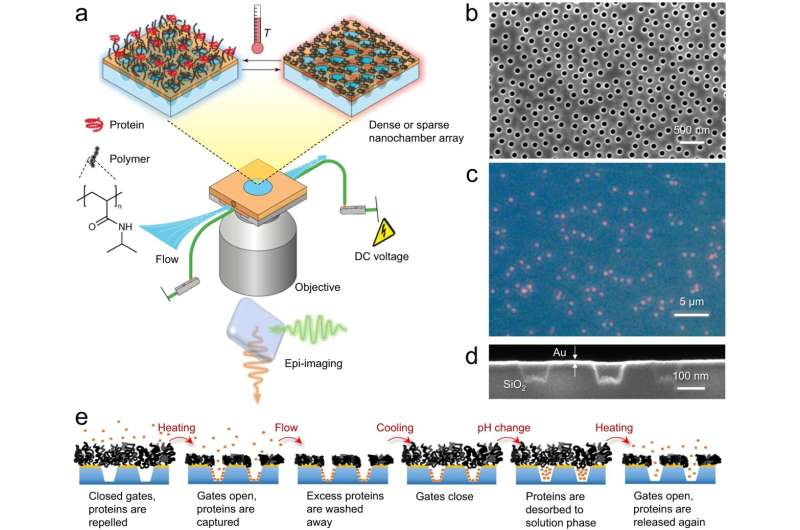
The researchers describe their work as the world's smallest gates that can be opened and closed at the touch of a button. The gates become traps, that lock the proteins inside chambers at the nanoscale. The proteins are prevented from escaping, extending the time they can be observed at this level from one millisecond to at least one hour. The new method also makes it possible to enclose several hundred proteins in a small volume, an important feature for further understanding.
"The clumps that we want to see and understand better consist of hundreds of proteins, so if we are to study them, we need to be able to trap such large quantities. The high concentration in the small volume means that the proteins naturally bump into each other, which is a major advantage of our new method," says Dahlin.
In order for the technique to be used to study the course of specific diseases, continued development of the method is required. "The traps need to be adapted to attract the proteins that are linked to the particular disease you are interested in. What we're working on now is planning which proteins are most suitable to study," says Dahlin.
How the new traps work
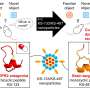
The gates that the researchers have developed consist of so-called polymer brushes positioned at the mouth of nano-sized chambers. The proteins to be studied are contained in a liquid solution and are attracted to the walls of the chambers after a special chemical treatment. When the gates are closed, the proteins can be freed from the walls and start moving towards each other.
In the traps, you can study individual clumps of proteins, which provides much more information compared to studying many clumps at the same time. For example, the clumps can be formed by different mechanisms and have different sizes and different structures. Such differences can only be observed if one analyzes them one by one.
In practice, the proteins can be retained in the traps for almost any length of time, but at present, the time is limited by how long the chemical marker—which they must be provided with to become visible—remains. In the study, the researchers managed to maintain visibility for up to an hour.
More information: Justas Svirelis et al, Stable trapping of multiple proteins at physiological conditions using nanoscale chambers with macromolecular gates, Nature Communications (2023). DOI: 10.1038/s41467-023-40889-4
Journal information: Nature Communications
Provided by Swedish Research Council