November 27, 2023 dialog
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
written by researcher(s)
proofread
Researchers describe the journey of thermal antibubbles in a hot bath
Bubbles are thin liquid shells surrounded by air. Although less well known, there are also antibubbles, which are the opposite of bubbles, i.e., a thin envelope of vapor surrounded by liquid. In a new study, we show that it is possible to create antibubbles by impacting a droplet of a volatile liquid on a bath of viscous oil heated to a temperature above the droplet's boiling point.
We discovered this phenomenon by serendipity at the laboratory GRASP at Université de Liège while studying another problem concerning the appearance of the Leidenfrost effect for a volatile droplet on a liquid bath.
During this study, we gently deposited the volatile droplet on a hot bath of viscous oil. The original idea was to reduce droplet movement as much as possible so as not to affect the measurement of the onset of the Leidenfrost effect. This effect, named after a German scientist of the 18th century, corresponds to the paradigmatic situation where a droplet of water moves on a hot pan, virtually without friction. The research is published in the journal Physical Review Letters.
Indeed, the heat provided by the pan vaporizes the droplet, thus effectively leading to its levitation above the hot surface. By extension, the Leidenfrost effect applies to any situation where an object is separated by a gas layer sustained by its own evaporation caused by a heat transfer from the substrate.
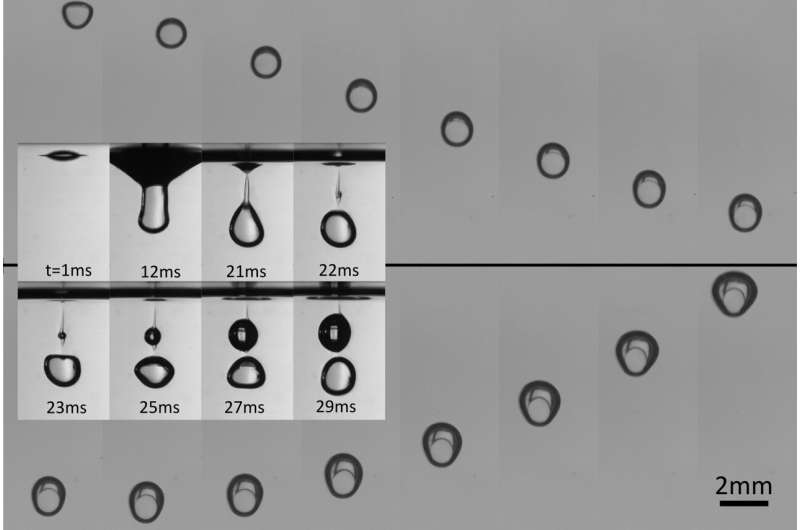
However, in the course of this study, we noticed that if we released the volatile droplet from higher, the droplet's kinetic energy allowed it to penetrate the bath, surrounded by a thin film of gas. The subsequent gas-coated column that is dynamically formed destabilizes and eventually pinches off. The result is a droplet encapsulated by a thin layer of vapor surrounded by the liquid bath, i.e., an antibubble.
Such objects were made before in isothermal conditions but their existence was extremely short, less than 100 ms. Indeed, since the hydrostatic pressure is higher at the bottom than at the top of the antibubble, a gravity-driven drainage fosters a flow of gas.
The bottom then becomes thinner, more fragile and eventually the droplet and the liquid in the bath come into contact, leading to the death of the antibubble. However, when a volatile droplet is used in an overheated bath, a flow of heat from the bath towards the droplet, through the thin gas shell, is set and the subsequent vaporization of the droplet can counteract the effect of drainage.
The resulting antibubble is much longer-lived. As the physical origin of these relatively stable antibubbles is the difference in temperature between the bath and the droplet, we coined the terminology "thermal antibubbles" for these objects.
As a first step, we systematically studied the impact conditions, i.e., the inertia of the incoming droplet, and the temperature difference between the bath and the droplet that led to the formation of thermal antibubbles. We established a phase diagram as a function of these two parameters where antibubbles can be created for the pair of fluids they considered in their study.
Then, we focused on the dynamics of a thermal antibubble after it formed. We observed that the antibubble first sinks in the bath as the density of the liquid composing the droplet is larger than the density of the viscous bath and the vapor layer surrounding the droplet is initially very thin. As the bath is hotter than the droplet's boiling point, the droplet evaporates and feeds the gas layer of the antibubble without boiling (that is the magic of Leidenfrost).
As a result of the vapor generation, the buoyancy of the antibubble increases and reaches a point where it equals the weight of the droplet and the antibubble stops. Subsequently, the buoyancy of the antibubble overcomes the weight of the droplet and its movement reverses towards the surface of the bath.
As the antibubble completes its journey through the hot bath, we track the contours of the antibubble and deduce its volume as a function of time. For a droplet of about 800 μm in radius and a temperature difference between the bath and the droplet close to 80°C, we observed that the volume of the antibubble increased by a factor of three in about 200 ms. For larger temperature differences, the inflation rate of the antibubble is shown to be even higher.
In order to rationalize their observations, our colleagues from the TIPs laboratory at University Libre de Bruxelles and who are involved in this study, worked to model the problem. Since the heat transfer that leads to the evaporation of the droplet is slaved to the thickness of the gas layer that is itself impacted by the gravitational drainage, a coupled model of heat and fluid transport must be written.
The first step was to adapt the models previously developed to rationalize the dynamics of the vapor layer in the problem of Leidenfrost droplets on a liquid substrate. But unfortunately, this approach predicted a much higher inflation rate of the antibubble, about 20 times higher than the one observed experimentally.
We worked hard to find the missing ingredient of this model. Finally, we found that the missing ingredient was the thermalization of the droplet at room temperature when impacting, and pumping thermal energy from the bath to reach its boiling temperature. The effect of droplet thermalization is generally neglected in problems involving Leidenfrost droplets, as it concerns the early droplet dynamics, whereas experiments mainly study the total lifetime of these droplets.
In the present problem of thermal antibubbles, we proved that droplet thermalization is essential for predicting their dynamics. In the absence of thermalization, the inflation rate of the antibubbles would be much larger, which would considerably reduce their lifespan and make these objects even more ephemeral than they really are.
An analytical solution for the diffusional thermalization of a sphere suddenly brought to a temperature different on its interface than at its center was available in the literature. Luckily enough, further simplification of the initial solution was possible thanks to the short time scales considered and the computation of the model could be attained easily.
An experimental proof of the importance of droplet thermalization is the faith of small satellite droplets that sometimes appear when the mother droplet is pinched off by the bath at the moment of impact. The inflation rate of these satellite droplets is much higher than the mother droplet. The difference is so great that the volume of the tiny antibubble can quickly reach the one of the large antibubble. This observation is a direct proof of the main role of droplet thermalization, as satellite droplets thermalize much faster than mother droplets due to their small size.
Indeed, only the thermalization term can rationalize this observation in the equations that describe the problem. At the end of the day, it turns out that within the first 100 milliseconds after its creation, a Leidenfrost droplet pumps approximately 95% of the heat coming from the bath to thermalize and not to evaporate, as could be concluded from existing models.
We concluded that thermal antibubbles are unique objects to directly visualize the evaporation rate of volatile droplets under different thermal conditions and the consequences of droplet thermalization.
In the future, these objects could be considered as small probes for estimating the thermal properties of fluids in different situations of practical interest. Finally, if the lifetime of these thermal antibubbles is indeed a few times larger than their isothermal counterparts, we have not yet achieved complete satisfaction. The limiting factor for these objects is the fact that after reaching the interface back due to their rapidly changing density, they look much like regular surface bubbles and cannot be considered as antibubbles anymore.
The next story on this subject should be written from gravitation-free environments, hopefully on larger time scales, thanks to an ESA-approved project for parabolic flights likely to happen in 2024.
This story is part of Science X Dialog, where researchers can report findings from their published research articles. Visit this page for information about ScienceX Dialog and how to participate.
More information: Jonas Miguet et al, Thermal Antibubbles: When Thermalization of Encapsulated Leidenfrost Drops Matters, Physical Review Letters (2023). DOI: 10.1103/PhysRevLett.131.184001
Journal information: Physical Review Letters
The team includes researchers working in the field of soft matter from three laboratories in Belgium and France. Benoid Scheid and Stéphane Dorbolo have made major contributions to the problem of isothermal antibubbles in the past. Laurent Maquet and Baptiste Darbois Texier have studied various problems involving the Leidenfrost effect. Jonas Miguet is a specialist in mass transfer in thin fluid films.All these skills put together have made it possible to rationalize the dynamics of these new objects, that we called "thermal antibubbles."