This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Researchers propose new strategy to improve efficiency for nanotherapeutic delivery in tumors
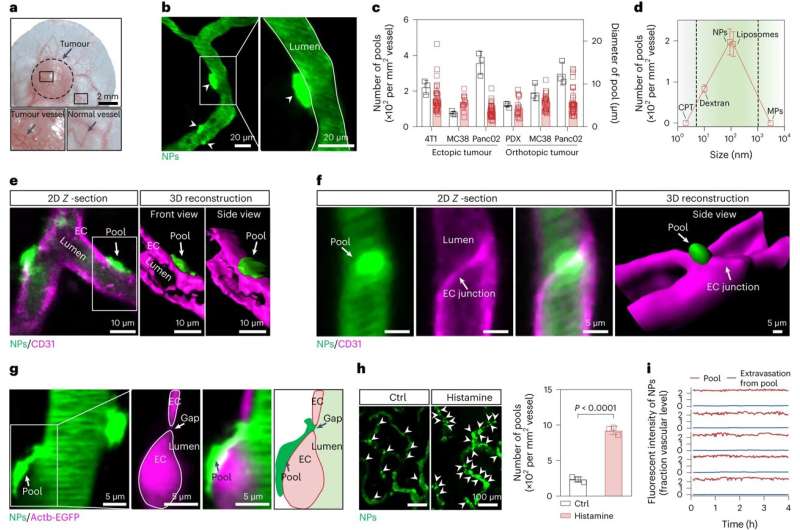
A team led by Prof. Wang Yucai and Associate Prof. Jiang Wei from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS) revealed the mechanism of the tumor vascular basement membranes (BM) blocking nanoparticles (NPs) for the first time and developed an immunodriven strategy to increase the NP penetration through the BM barrier. Their work was published in Nature Nanotechnology.
Previous research on the nanotherapeutic transport from the vasculature to the tumor mainly depended on the Enhanced Permeability and Retention effect (EPR), which believes that NPs can cross the tumor vascular endothelial barrier, the last defense of NP penetration, by exploiting the high permeability of tumor vessels. However, clinical trials discovered that NPs only transport around 0.7% of drugs into the tumor issue, suggesting other mechanisms for hindering NP penetration.
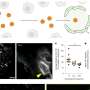
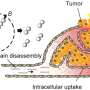
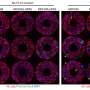
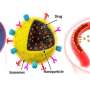
To shed light on this underappreciated mechanism, the team employed multistep non-invasive intravital microscopy and revealed that the BM that surrounds the endothelial cells and mural cells of tumor vessels severely impede the extravasation of NPs, forming perivascular NP pools in subendothelial void.
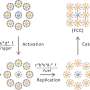
After accurately analyzing the spatial positioning, microstructure and causes of the NP pools, the team further found enzyme degradation of the BM could significantly reduce the NP pooling, boosting the transport efficiency of nanomedicine. Based on this finding, the team developed an immunodriven strategy by using the localized proteolytic enzymes released by inflammatory leukocytes to create a temporary window on the BM, enabling an explosive release of NPs deep into tumor, significantly enhancing the enrichment of nanomedicines and therapeutic effect.
The study not only proposes a novel nanomedicine transport strategy distinct from EPR, but also provides a new theoretical support for the application of nanotherapeutics in cancer, advancing the understanding of transvascular transport mechanism of NPs.
More information: Qin Wang et al, Breaking through the basement membrane barrier to improve nanotherapeutic delivery to tumours, Nature Nanotechnology (2023). DOI: 10.1038/s41565-023-01498-w
Journal information: Nature Nanotechnology
Provided by University of Science and Technology of China