This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Immune cell map reveals origin of subcellular response to microbes
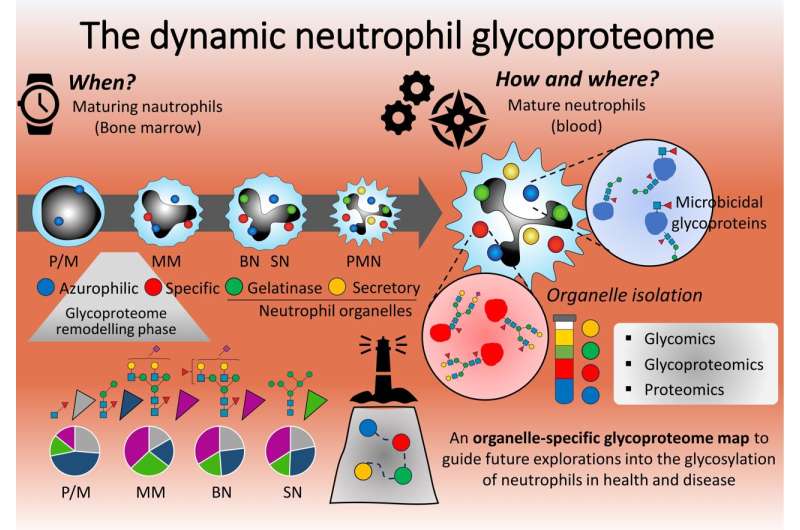
The first line of defense in our immune systems are white blood cells, 40% to 70% of which are neutrophils. These cells rush to sites of injury or infection, producing proteins to promote inflammation and attack invading microbes. At the time of response, the proteins are decorated with carbohydrate molecules—called glycoproteins—yet scientists do not know when or how these complex molecules appear.
Now, however, an international research team may be a step closer to understanding when and how neutrophils arm their microbe-killing proteins. The finding, the researchers said, may have implications for refining personalized immune responses.
They published their work in the Proceedings of the National Academy of Sciences.
"While we know that the microbicidal proteins produced by neutrophils are heavily modified by functionally relevant complex carbohydrates called glycans, we still do not understand exactly how, when and where the neutrophil proteins are glycosylated," said corresponding author Morten Thaysen-Andersen, Associate Professor, School of Natural Sciences, Macquarie University in Australia.
Thaysen-Andersen is also affiliated with Nagoya University's Institute for Glyco-core Research (iGCORE) in Japan. "Such details are important to better describe the molecular basis for key innate immune processes including pathogen defense. This paper makes use of new analytical tools in system biology to explore this critical knowledge gap."
Neutrophils circulate in the blood in a resting state, with what researchers call an "arsenal of prepackaged cytosolic granules," which are subcellular compartments adorned with anti-microbial glycoproteins. These subcellular compartments are mobilized under specific environmental cues, allowing the neutrophils to move to where they are needed and react as needed for that specific threat.
To understand how the neutrophils deploy the correct course of action, the researchers first isolated neutrophils from human blood donors. From the neutrophils, the researchers isolated the intracellular cytosolic granules. They then comprehensively profiled the entire complement of glycan-decorated proteins from these compartments.
By analyzing four key types of intracellular organelles in neutrophils across the cells' maturation stages from bone marrow to blood, the researchers could map how, when and where the neutrophil proteins present glycans to combat microbes and carry out other key inflammatory processes.
"Our study found that microbicidal proteins produced by neutrophils are richly decorated with highly unusual glycans," Thaysen-Andersen said. "Interestingly, the unusual glycosylation patterns were restricted to a particular compartment within the neutrophils hosting most microbicidal proteins."
According to researchers, their analysis indicated that a well-timed and coordinated expression of a specific type of glycoprotein early in the neutrophil's maturation was responsible for their restricted subcellular origin. The specific glycoprotein expression was coordinated with the initial development of the organelle that deploys a microbicidal response.
The finding, Thaysen-Andersen said, provides a "valuable resource" to guide future studies on the biological roles and potential dysregulation of neutrophil glycosylation.
"Guided by our high-precision map of healthy neutrophils, future glycoprofiling studies are now well positioned to investigate the glycosylation of immature neutrophils from individuals exhibiting emergency granulopoiesis—or the genesis of new neutrophils to respond to acute infection or septic shock—and patients with acute myeloid leukemia," Thaysen-Andersen said. "Such efforts hold an untapped potential for the discovery of glycoprotein-related immune responses under both normal and aberrant physiological conditions."
More information: Rebeca Kawahara et al, Glycoproteome remodeling and organelle-specific N -glycosylation accompany neutrophil granulopoiesis, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2303867120
Journal information: Proceedings of the National Academy of Sciences
Provided by Tokai National Higher Education and Research System





















