August 30, 2023 dialog
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
written by researcher(s)
proofread
Nanocomposite-based electrocatalyst for alkaline overall water splitting at low cell voltage for hydrogen production
The diminishing conventional energy resources based on fossil fuels and their related environmental consequences have drawn attention around the world toward the development of renewable energy resources. These renewable energy resources may not fulfill the entire energy demands of world's mass population; however, they limit the effects of greenhouse gases as well as air pollution caused by the fossil fuel burning. Among alternative resources, hydrogen is considered to be the cleanest energy carrier.
However, hydrogen does not exist in its pure state in nature, like oxygen, and has to be produced from hydrogen-containing resources such as natural gas (methane), coal, biomass and water by reforming, thermal decomposition or electrolysis. But production of hydrogen from natural gas, coal and biomass leads to the emission of the greenhouse gas carbon dioxide (CO2).
We know that water (H2O) is made of hydrogen and oxygen atoms; hence, sea water could be a limitless source of hydrogen. Therefore, hydrogen is envisaged as a possible replacement for fossil fuels. Production via power from renewable energy (using wind power, solar power, hydropower, wave power or similar) is termed as "green hydrogen." In this scenario, splitting water into hydrogen and oxygen using renewable electricity in an electrolyzer on the surface of a robust electrocatalyst is one proposed technique.
Need for a robust electrocatalyst
Despite advancements in the field, the process of water spitting to produce affordable green hydrogen still remains sluggish due to limitations related to efficient electrocatalysts. In theory, water splits at 1.23 V. However, in practical terms, this value is greater than 1.5 V (meaning wastage of additional energy). This minimum energy is theoretically required to break the water molecule. Expensive noble- and precious-metal-based electocatalysts, for instance, Pt, Pd, Au, Rh, Ir, etc., are used in the electrolyzer for this process.
The main problems the industry and experts face are the oxidation of water to produce O2 and the stability of the catalyst in harsh industrial alkaline conditions. In the first problem, the half-cell reaction is an uphill reaction where four electrons are involved and where most of the energy is required apart from the energy loss associated with the resistivity of different components (electrolyte, connections, catalyst, etc.) of the electrolyzer. In the second problem, the expensive catalysts often lose their activity due to surface degradation. In these conditions, a cheap and affordable yet highly active and stable electrocatalyst is required for such water splitting reaction.
Present development
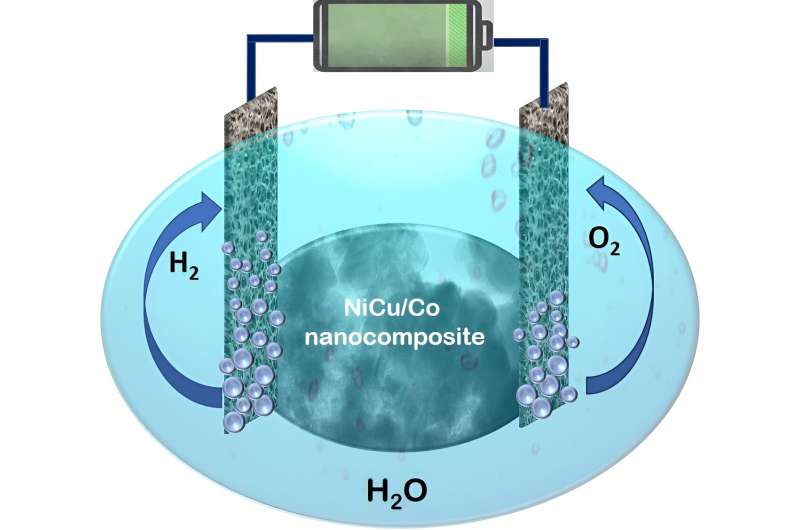
In a recent study, our team, led by Sasanka Deka, designed and developed a new nanocomposite-based, highly efficient, yet still cost-effective electrocatalyst for overall water splitting. A nanocomposite is a homogeneous mixture of two or more materials present in the nanometer range. The present nanocomposite is a nanoarchitecture based on NiCu dealloyed nanoparticles on hierarchical Co nanosheets. Our findings are published in the journal ACS Catalysis.
The materials used are cheaper than the precious metals and the synthesis procedure is highly convenient. This new catalyst was used in an electrolyzer in potassium hydroxide (KOH) electrolyte for the splitting of water. Interestingly, the system shows the splitting of water and production of hydrogen gas using the NiCu/Co electrocatalyst at 1.46 V cell voltage. Thus, the electrocatalyst is capable of splitting of water by using only a household 1.5-volt battery.
Other key points about the NiCu/Co electrocatalyst are the green hydrogen production takes place with industrially important high current density, high stability (6,000 cycles) and durability (60 h) of the catalyst. It also works on industrial electrolyte condition of 30 wt.% KOH electrolyte and the cell voltage offered is much lower than that of a commercial IrO2||Pt/C catalyst.
Detailed experimental and computational studies have been performed to understand the reason behind this efficiency. The corroborated results support our initial hypothesis of selective leaching of materials to make a more porous structure, and the use of different metal centers and shapes of materials for hydrogen and oxygen evolution.
In summary, we have developed a simple but advanced and cost-effective method to design a nanocomposite-based, bifunctional electrocatalyst of dealloyed NiCu on Co nanosheets that can split water at 1.46 V with great stability. We hope that our product could be useful for scale-up synthesis and commercial use in electrolyzers for green hydrogen production.
This story is part of Science X Dialog, where researchers can report findings from their published research articles. Visit this page for information about ScienceX Dialog and how to participate.
More information: Ankur Kumar et al, Designing Nanoarchitecture of NiCu Dealloyed Nanoparticles on Hierarchical Co Nanosheets for Alkaline Overall Water Splitting at Low Cell Voltage, ACS Catalysis (2023). DOI: 10.1021/acscatal.3c02096
Journal information: ACS Catalysis
Dr. Sasanka Deka is Professor of Chemistry, University of Delhi. He received his Ph.D. degree from National Chemical Laboratory (NCL-Pune). He did his postdoctoral research from National Nanotechnology Laboratory, CNR-INFM, Lecce, Italy and Italian Institute of Technology (IIT), Genova, Italy. He has been awarded the TMS Foundation 2008 SHRI RAM ARORA AWARD, by the Minerals, Metals & Materials Society (TMS), Warrendale, USA; DAE-BRNS Young scientist research award 2011, RSC best oral talk–2015, Institute of Physics (IOP), UK best cited paper-India 2019 and RSC best cited paper in 2020. Dr. Deka has published more than 75 research papers in different international high impact journals, holds three patents, and also wrote two books and three book chapters published by an international publisher. He has successfully handled several extramural national and international research projects. His current research interest deals with synthetic nanochemistry, and novel nanomaterials for energy research.