This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A scalable, safer, and potentially cheaper way to isolate valuable isotopes
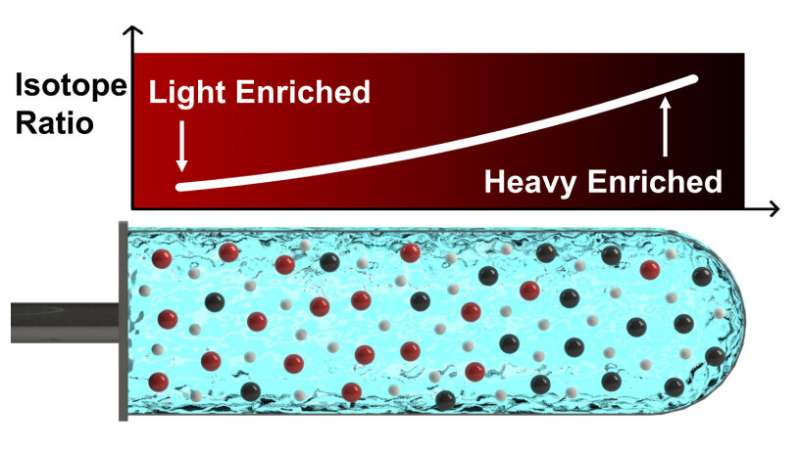
New research published in Science Advances, led by Yuan Yang, associate professor of materials science at Columbia Engineering, and collaborators at Lamont-Doherty Earth Observatory, demonstrates a novel technique for isolating isotopes.
Oxygen is a critical component in the positron emission tomography (PET) scans oncologists use to search for tumors. But not just any oxygen will work. While most oxygen atoms have eight neutrons, about 1 in 500 atoms has ten. Those extra neutrons are necessary for the PET imaging scans to work.
It's extremely expensive to isolate the slightly heavier oxygen atoms. A cubic meter of regular water (H2O) costs less than $2 from your tap. When the lighter oxygen atoms (and hydrogen) are removed, the heavier oxygen atoms that remain are worth closer to $30,000.
Researchers at Columbia figured out how to isolate heavier or lighter atoms—called isotopes—by dissolving the target element and salts in water before spinning that solution in a centrifuge. It's more effective, cheaper, and easier to scale than the current state-of-the-art techniques, which also use toxic chemicals that aren't necessary for the new method.
Pure isotopes are extremely useful—and extremely valuable. Every year, tens of millions of people across the world receive medical tests that require a hard-to-extract isotope called 100-Molybdenum. Calcium isotope Ca-48 is so rare and sought-after that a single gram currently costs $500,000. And if nuclear fusion becomes a viable source of energy, it will take thousands of tons of a lithium isotope to satisfy the world's energy demands.
Since collaborating with Columbia Technology Ventures to patent the new technology, the researchers have been in touch with several companies about building prototypes and developing a plant to begin isolating isotopes. They've used computational modeling to discover several innovative methods that further increase the method's efficiency.
More information: Joseph F. Wild et al, Liquid solution centrifugation for safe, scalable, and efficient isotope separation, Science Advances (2023). DOI: 10.1126/sciadv.adg8993
Journal information: Science Advances





















