This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Scientists adopt high entropy perovskite and quaternary alloy to enable high-efficiency carbon dioxide reduction
The pressing economic and political challenges faced by countries worldwide have now shifted towards addressing the critical issue of global warming caused by excessive carbon dioxide (CO2) emissions. Consequently, there is a growing focus on the application of CO2 reduction reaction (CO2RR) through solid oxide electrolysis cells (SOECs), which have the capability to convert CO2 into valuable carbon monoxide (CO) using electricity generated from advanced renewable power generation technologies such as tidal energy, geothermal energy and solar energy.
"Developing a highly efficient electrode suitable for use in both anode and cathode is crucial in SOECs commercial application," said the study's co-corresponding author Yao Wang, an associate professor at the School of Power and Mechanical Engineering at Wuhan University. "The ultimate goal is to simplify the technique and to reduce costs—demands that the high entropy system and alloy nanocatalyst can meet precisely."
In pursuit of this objective, Wang and colleagues have successfully developed an innovative high-entropy perovskite-type symmetrical electrode for SOECs. This electrode incorporates Fe-Co-Ni-Cu quaternary alloy nanocatalysts derived from the cathode in a reducing atmosphere, leading to remarkable catalytic activity for CO2RR.
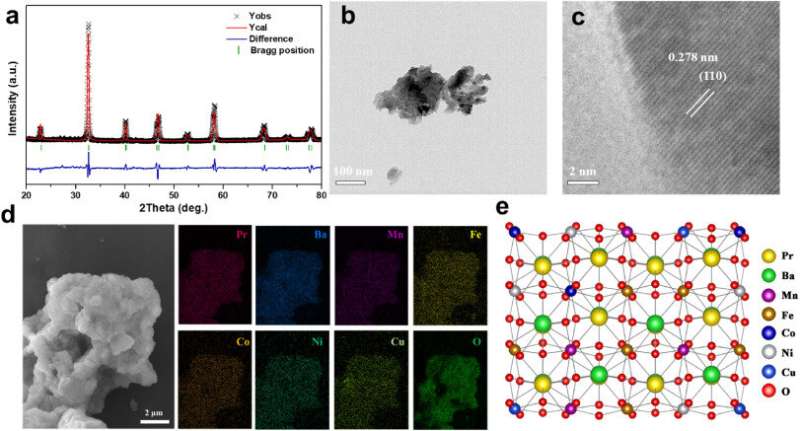
"We successfully synthesized a high-entropy perovskite oxide, namely, Pr0.5Ba0.5Mn0.2Fe0.2Co0.2Ni0.2Cu0.2O3-δ (HE-PBM), by leveraging the properties of Pr0.5Ba0.5MnO3-δ material. This unique composition combines the advantages of transition elements to enhance the oxygen evolution reaction (OER) at the anode. Furthermore, we observed the in-situ formation of Co-Fe-Ni-Cu quaternary alloy nanoparticles, which further contributed to the performance of the material," explained Wang.
Compared with the commonly used monometallic or two-phase alloy particles, this research accomplishes the in-situ exsolution of quaternary alloys through reduction treatment. The team's approach leads to a remarkable enhancement in both CO2 adsorption and electrochemical catalytic performance.
"The electrochemical evaluation of the material's performance in CO2RR demonstrates exceptional results, with a high current density of 1.21 A cm-2. Importantly, the material exhibits excellent stability over prolonged operation, showing no signs of decay or coking," added Mingyue Ding, the other co-corresponding of the study.
The team published their findings, which will undoubtedly open up new avenues and perspectives for achieving efficient and sustainable CO2 electrolysis, in the journal Advanced Powder Materials.
More information: Dong Zhang et al, Novel high-entropy perovskite-type symmetrical electrode for efficient and durable carbon dioxide reduction reaction, Advanced Powder Materials (2023). DOI: 10.1016/j.apmate.2023.100129
Provided by KeAi Communications Co.