April 17, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
The comprehensive characterization of hydrogen at ultra-high pressures
Physicists and material scientists have been trying to metallize hydrogen for many decades, but they have not yet succeeded. In 1968, British physicist Neil Ashcroft predicted that atomic metallic hydrogen would be a high-temperature semiconductor.
Most recent studies also suggested that this elusive and hypothetical form of hydrogen would also conduct electricity with no resistance when its temperature exceeds that of boiling water. This prediction ultimately paved the way for the discovery of high-temperature superconductivity in hydrides (i.e., compounds containing hydrogen and a metal).
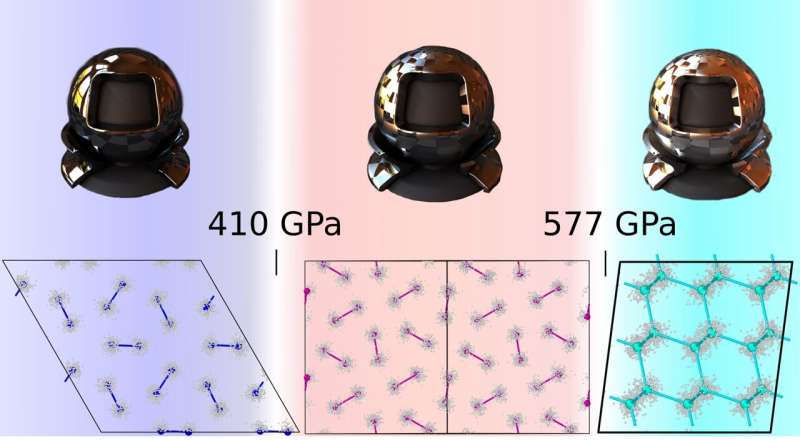
Researchers at Sapienza University of Rome, Sorbonne University, CNRS, and the International School for Advanced Studies (SISSA) have recently carried out a study aimed at thoroughly characterizing the behavior and properties of hydrogen at high pressures. Their paper, published in Nature Physics, outlines a highly accurate phase diagram of high-pressure hydrogen, which could inform ongoing efforts aimed at creating atomic metallic hydrogen.
"The most advanced laboratories worldwide are trying to synthesize stable atomic metallic hydrogen by compressing hydrogen molecules into diamond anvil cells up to pressures millions of times higher than the atmospheric one," Lorenzo Monacelli, one of the researchers who carried out the study, told Phys.org. "However, the H2 covalent bond is one of the strongest in chemistry, and the pressure required to push hydrogen nuclei close enough to break the molecular bond is far higher than expected. "Our work rationalizes the latest experimental findings and reports the most accurate determination of the high-pressure hydrogen phase diagram."
Reliably studying the behavior and properties of hydrogen at high pressures is a challenging task, as the necessary experiments are difficult to perform, while standard simulation tools often yield unreliable results. Monacelli and his colleagues used advanced computational tools to create a phase diagram of hydrogen and deuterium at low temperatures and high pressures.
"At such high pressure, many different molecular phases are stable," Michele Casula, another researcher involved in this study, explained. "It is paradoxical and fascinating to discover how complex the phase diagram is despite the simplicity of its elementary brick, made of a proton and an electron only. In some sense, it is a particularly significant example of Phill Anderson's famous motto: 'More is different.'"
Solid hydrogen is fully comprised of free electrons and protons that interact with each other. A crucial challenge when trying to simulate hydrogen is that the quantum behavior of these two types of particles creates complex interactions.
"The quantum behavior of electrons and protons is different, as each proton weighs about 2,000 times the electron," Casula said. "In our work, we employed the 'best' method to solve the electronic problem (i.e., quantum Monte Carlo simulations) and the best approach to solve the proton quantum effects, namely the stochastic self-consistent harmonic approximation."
Using these state-of-the-art computational techniques, the researchers were able to create one of the most accurate phase diagrams of high-pressure hydrogen to date. Specifically, they showed that the experimentally elusive atomic metallic hydrogen phase should form at 577(4) GPa, after another stage in which molecular hydrogen transitions from one metallic phase (referred to as phase III) to another phase in which the hydrogen is metallic and still molecular (phase VI).
"The comprehensive characterization of this material at ultra-high pressures provided by our work suggests that the atomic phase, supposed to be a room-temperature superconductor, must be searched experimentally at much higher pressures than previously suggested," Casula explained. "Moreover, our study allowed the interpretation of recent experimental results in apparent contradiction, as researchers can indirectly probe the crystal structure at such high pressures."
The new phase diagram of high-pressure hydrogen computed by this team of researchers suggests that hydrogen starts acquiring metallic properties progressively as the pressure rises. It also shows why previous experiments may have been unsuccessful in attaining atomic metallic hydrogen, namely because the pressures tested in experiments were not high enough.
In their paper, Monacelli, Casula and their colleagues also present a complete and exhaustive spectroscopic characterization of hydrogen. Together with the phase diagram they created, this characterization could inform future research efforts aimed at observing hydrogen in its atomic metallic phase.
"Notably, we predicted a clearcut isotope shift for the transition pressures, measured and confirmed very recently," Monacelli added. "We are now focusing on studying the superconductive properties of hydrogen at high pressure. This problem is exciting and presents critical challenges, as the current theory of superconductivity based on the interaction between lattice vibrations (phonons) and electrons misses some crucial aspects present in hydrogen and hydrides."
Monacelli and Casula pointed out that the current theory of superconductivity does not account for strong anharmonic propagations of phonons, the nonlinear coupling between phonons and electrons, and the dynamic interplay between electrons and fast nuclei (i.e., the "adiabatic" approximation). In collaboration with their colleagues, they aim at devising a new theory that collectively considers all these phenomena. In addition, the researchers are now planning to create detailed characterizations of other superconductive hydrides using the same computational tools employed in their recent study.
More information: Lorenzo Monacelli et al, Quantum phase diagram of high-pressure hydrogen, Nature Physics (2023). DOI: 10.1038/s41567-023-01960-5
Journal information: Nature Physics
© 2023 Science X Network