This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Producing synthetic gas from atmospheric carbon dioxide using a transition-metal-free catalyst
Japan has declared carbon neutrality by 2050 as a government target to reduce net greenhouse gas emissions to zero by 2050. In order to substantially reduce CO2 emissions, it is important not only to control CO2 emissions through existing processes, but also to develop innovative processes to utilize CO2 as a resource for production of fuels and chemicals. The core of these efforts is technology to produce syngas, a carbon monoxide and hydrogen gas mixture, from CO2.
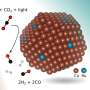
Syngas is produced by using a reverse water gas shift reaction (CO2+H2→CO+H2O) that reduces CO2 to carbon monoxide by reaction with hydrogen. A highly active transition metal such as platinum, rhodium, nickel, or copper has been considered necessary as a catalyst component to facilitate this reaction.
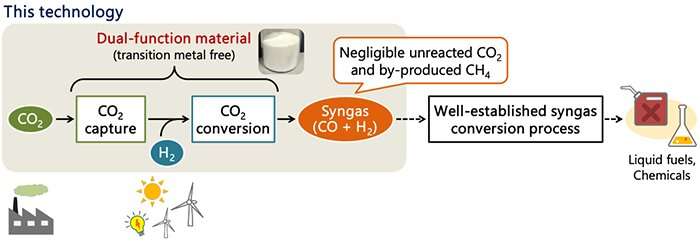
Prior to the reaction operation, the energy- and cost- intensive processes were necessary to separate and recover CO2 by a chemical absorption technique and subsequently purify it close to 100 %. An innovative technology, which can directly produce syngas from low-concentration CO2 without these processes, needs to be developed. Recently, dual-function materials have been attracting attention to enable integrated CO2 capture and conversion.
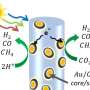
In collaboration with Delft University of Technology, researchers in AIST developed a technology for directly producing syngas that is a highly versatile raw material for fuels and chemicals. This technology can utilize CO2 in exhaust gases from power plants and industrial sectors (~20 %) as well as the low-concentration atmospheric CO2 (400 ppm; 0.04 %) to produce syngas.
This technology can directly produce syngas, a carbon monoxide and hydrogen gas mixture, by using the dual-function materials to react low-concentration CO2 with hydrogen derived from renewable energy, without separation and purification processes. While the conventional reaction to reduce CO2 to carbon monoxide required a catalyst using a transition metal, this technology employs the transition-metal-free dual-function material which has a simple composition with an alkali or alkaline earth metals such as sodium.
In contrast to the conventional method, this technology hardly produces unreacted CO2. Moreover, a crucial factor in the syngas production namely the H2/CO ratio is anticipated to be controllable by varying operating conditions such as the hydrogen flow rate.
The work is published in the Journal of CO2 Utilization.
More information: Tomone Sasayama et al, Integrated CO2 capture and selective conversion to syngas using transition-metal-free Na/Al2O3 dual-function material, Journal of CO2 Utilization (2022). DOI: 10.1016/j.jcou.2022.102049
Provided by Advanced Industrial Science and Technology