This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Using light to switch drugs on and off
Scientists at the Paul Scherrer Institute PSI have used the Swiss X-ray free-electron laser SwissFEL and the Swiss Light Source SLS to make a film that could give a decisive boost to developing a new type of drug.
They made the advance in the field of so-called photopharmacology, a discipline that develops active substances which can be specifically activated or deactivated with the help of light. The study was published on Feb. 17 in the journal Nature Communications.
Photopharmacology is a new field of medicine that is predicted to have a great future. It could help to treat diseases such as cancer even more effectively than before. Photopharmacological drugs are fitted with a molecular photoswitch. The substance is activated by a pulse of light, but only once it has reached the region of the body where it is meant to act. And after it has done its job, it can be switched off again by another pulse of light.
This could limit potential side effects and reduce the development of drug resistance—to antibiotics, for example.
Light-switchable drugs
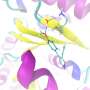
To make conventional drugs sensitive to light, a switch is built into them. In their study, the scientists led by the principal authors Maximilian Wranik and Jörg Standfuss used the active molecule combretastatin A-4, which is currently being tested in clinical trials as an anti-cancer drug. It binds to a protein called tubulin, which forms the microtubules that make up the basic structure of the cells in the body, and also drive cell division. Combretastatin A-4, or "CA4" for short, destabilizes these microtubules, thereby curbing the uncontrolled division of cancer cells, i.e., it slows down the growth of tumors.
In the modified CA4 molecule, a bridge consisting of two nitrogen atoms is added, which makes it particularly photoactive. In the inactive state, the so-called azo bridge stretches the molecular components to which it is attached to form an elongated chain. The pulse of light bends the bond, bringing the ends of the chain closer together—like a muscle contracting to bend a joint.
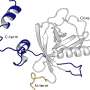
Crucially, in its elongated form, the molecule does not fit inside the binding pockets of the tubulin—depressions on the surface of the protein where the molecule can dock in order to exert its effect. However, when the molecule is bent, it fits perfectly—like a key in a lock. Molecules like this, which fit into corresponding binding pockets, are also called ligands.
Filming a possible cancer drug
The latest study shows that the processes involved go well beyond the simple lock-and-key principle. "Contrary to what the textbooks say, both the key and the lock behave dynamically; they are constantly changing their shape," says Maximilian Wranik. "The entire protein is anything but static."
Often, the binding pockets are only half open, the ligand lodges in them briefly and is released again before it can do its job. Alternatively, you can have a so-called "induced fit," where something that isn't really the right shape is "made to fit." The ligand alters the shape of the pocket such that it can get properly lodged in place and remain there.
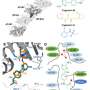
The scientists have now filmed the ligand in the binding site, as it changes from the bent configuration to the straight shape after being switched off, showing how the pocket adapts somewhat to this new configuration before the ligand detaches itself. The binding pocket then collapses and, after a while, re-forms. What is clear is that the better the ligand fits, the longer it remains bound to the site.
In any case, a more thorough understanding of these processes, which have been made visible for the first time, opens up the possibility of designing new active substances with a better fit, so that the binding time and thus the effectiveness of a drug can be improved.
A new level of structure determination
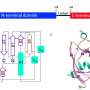
The processes involved take place on an atomic level, and within milliseconds. In order to observe them, the researchers used the high-precision large research facilities at PSI, the combination of which is unparalleled anywhere in the world: the Swiss Light Source SLS and the Swiss X-ray free-electron laser SwissFEL can not only record individual images on miniscule timescales and tiny dimensions, but an entire sequence of images which can then be assembled to create a film.
"We took nine snapshots between one nanosecond and 100 milliseconds after the active molecule had been switched off," says Jörg Standfuss, the project leader. The photobiologically relevant processes take place during this period.
Among other things, his team used SLS to analyze the structure of the molecules involved, down to the atomic level, and SwissFEL to measure the processes to within 100 femtoseconds, or a tenth of a trillionth of a second. "Without the excellent support and collaboration with the experts at SwissFEL and the SLS, the realization of such a unique project would not have been possible," emphasizes Standfuss.
Filming active substances against gout or COVID-19
The possibility of filming photoactive substances at work also opens up the opportunity of gathering many other important insights in the field of medicine. "Of course, we'd also like to track the exact sequence of events when the active substance is switched on," says Standfuss. "That's a bit more complicated, though—so we won't be tackling that until the next stage."
Aside from this, the study only looks at one of many known binding pockets in tubulin. Even this one does not simply serve as a docking site for cancer drugs. Colchicine, which is used to treat gout and other inflammatory rheumatic diseases, and the new COVID 19 drug sabizabulin, which is still under development, also bind to the same pocket. The new method could therefore be used to look at other drugs or other binding sites.
The hope is that this method will help clinical research to come up with more effective therapies for a wide variety of diseases, says Standfuss. "With the help of our large research facilities, we want to open up time as a new dimension when determining the structure of active substances, enabling us to understand them and optimize them even further."
More information: Maximilian Wranik et al, Watching the release of a photopharmacological drug from tubulin using time-resolved serial crystallography, Nature Communications (2023). DOI: 10.1038/s41467-023-36481-5
Journal information: Nature Communications
Provided by Paul Scherrer Institute