This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Prion disease: PRNP sequences of wild animals from the Qinghai-Tibet Plateau
Tibetan antelope (Rhinopithecus), blue sheep (Pseudois nayauris), and plateau pika (Ochotona curzoniae) are wild animals living on the Qinghai-Tibet Plateau. There have been no reports of naturally occurring transmissible spongioform encephalopathies (TSEs) involving these animals. Furthermore, the PRNP genes have not been described in the literature.
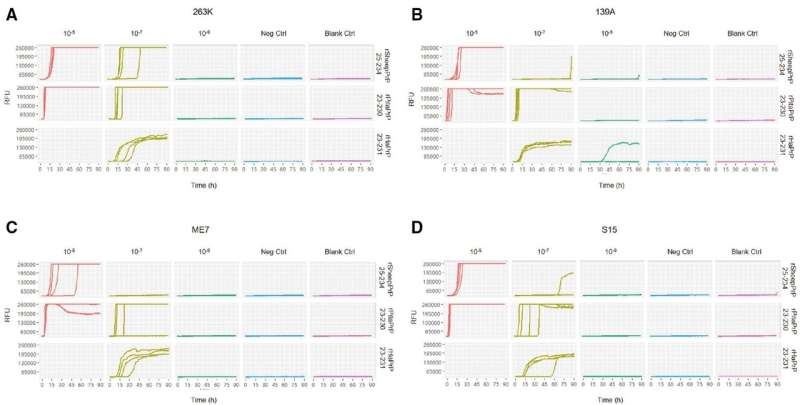
For a study published in Zoonoses, the PRNP genes from 21 Tibetan antelopes, four blue sheep, and three plateau pikas were obtained and sequenced. The recombinant proteins were then prepared. Using scrapie strains (263K, 139A, ME7, and S15) as the seeds, the reactivity of the PrP proteins from sheep (rSheepPrP25-234) and pika (rPikaPrP23-230) were tested using real-time quaking-induced conversion (RT-QuIC). Protein misfolding cyclic amplification (PMCA) tests of the brain homogenates from domestic sheep and rabbits were performed with the seeds of strains 263K and ME7.
The PRNP genes of bovids were 771 bp long and encoded 256 amino acids (aa), showing 100% homology with the wild-type sheep prion protein (PrP) aa sequence. The PRNP gene of pika was 759 bp long and encoded 252 amino acids, showing 92.1% homology with the aa sequence of domestic rabbits. The sheep and pika proteins revealed positive reactions in 10-5 diluted seeds. Only rPikaPrP23-230 produced positive curves in 10-7 diluted seeds. The PMCA tests failed to produce proteinase K (PK)-resistant PrP (PrPres).
This is the first description of PRNP genes and PrP aa sequences of Tibetan antelope, blue sheep, and plateau pike from the Qinghai-Tibet Plateau. In the presence of rodent prions, the PrPs of sheep and pika efficiently induce fibrillation in RT-QuIC, but do not generate PrPres in PMCA. Our results indicate that pika, as one of the important links in the Qinghai-Tibet Plateau biological chain, may play an important role in the prion circulation. Pika PrP deserves further analysis for its potential application value in assays for human prion disease.
More information: Yue-Zhang Wu et al, PRNP Sequences of Tibetan Antelope, Blue Sheep, and Plateau Pika from the Qinghai-Tibet Plateau and Reactivity of PrP Proteins to Rodent-Adapted Scrapie Strains in RT-QuIC and PMCA, Zoonoses (2023). DOI: 10.15212/ZOONOSES-2022-0036
Provided by Compuscript Ltd