A new protocol for light-sheet live imaging of C. elegans adults
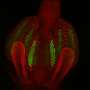
The beauty of live-imaging studies is the specimen is alive, allowing dynamics such as cell division and embryonic development to be recorded over time.
Yet the frustration of live-imaging studies is the specimen is alive—wriggling, twisting, escaping the field of view. Plus, it's delicate, susceptible to heat damage or death from the imaging equipment itself.
A technical solution to this quandary recently emerged from the MBL Embryology course, in "a classic example of the collaborative effort here at MBL," says MBL Imaging Research Specialist Carsten Wolff.
"During the 2021 Embryology course, we started to develop a technique that enables us to image adult C. elegans worms for longer periods of time, and at high resolution, using light sheet microscopy," says Wolff. A group of course faculty and staff, collaborating with MBL imagers, fine-tuned the protocol during the 2022 course and wrote up the paper, which is published this month in Frontiers in Cell and Developmental Biology.
The nematode C. elegans is a popular organism in biological and biomedical research. Light-sheet fluorescence microscopy (LSFM) has been very successful in capturing embryonic processes in C. elegans, as well as in mice and zebrafish. But once the organisms hatch out, LSFM presents limitations.
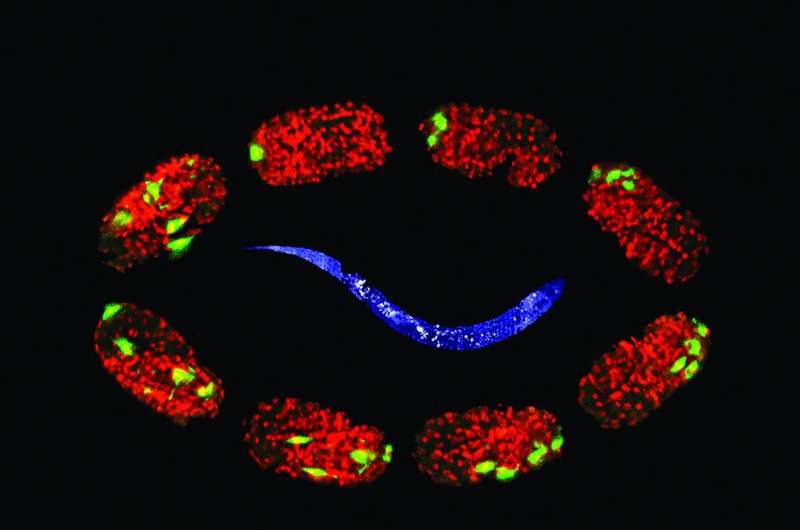
In C. elegans, the difficulty had been sample mounting. Due to its optical properties, low-melt agar works well as a sample medium for larger organisms, but the little roundworms tend to burrow into the soft agar and disappear. Consequently, prior to this protocol, the longest LSFM imaging time for adult C. elegans had been 20 minutes. The new protocol extends that time to more than two hours, while avoiding heat stress in the specimen.
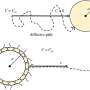
"The innovation we describe is essentially a combination of two known mounting approaches," Wolff says.
"One is a biopolymer that is viscous during sample preparation, but once you expose it to UV light it hardens and keeps the sample (in this case, C. elegans) immobile. The second part is a mounting method in plastic tubes that allows the use of light sheet microscopy. The combination allows one to live-image adult C. elegans over a period of more than 2 hours."
"It sounds like a short time period, but because of previous problems with immobilizing the specimen, this was not possible. Also, imaging from different angles, which LSFM allows, wasn't possible before because of the specimen's constant body movement."
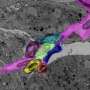
The team used the protocol to time-lapse image a sensory neuron's dendrites branching and pruning. And they expect it will enable better live-imaging studies of other important cell and developmental processes, such as germ stem cell biology, cell migration, cell division and cell invasion. The protocol is generalizable to work with other organisms, with little or no modifications.
More information: Jayson J. Smith et al, A light sheet fluorescence microscopy protocol for Caenorhabditis elegans larvae and adults, Frontiers in Cell and Developmental Biology (2022). DOI: 10.3389/fcell.2022.1012820
Provided by Marine Biological Laboratory