Effect of cation types on electrochromic properties of titanium dioxide nanocrystals
Electrochromic (EC) devices have been regarded as promising candidates for energy-saving smart windows, next-generation displays, and wearable electronics. Monovalent ions such as H+ and Li+ based electrolytes are the benchmark insertion ions for EC devices but have serious limitations such as high cost and instability coupled with being difficult to handle. Seeking multivalent electrolytes is an effective alternative to prepare high-performance EC devices; unfortunately, the related reports are currently limited to tungsten oxide EC materials. Researchers in China unravel the effect of different valence cations on the EC properties of titanium dioxide nanocrystals (NC), which may provide some new directions for the development of excellent EC devices with long-term stability and durability.
They published their work on August 3 in Energy Material Advances.
"It is of great significance to search for alternative cheap, stable, and rapid insertion ions in EC devices to achieve cost-effective and rapid EC application," said corresponding author Sheng Cao, associate professor with the School of Physical Science and Engineering Technology, Guangxi University. "Currently, there are many EC materials with Li+ and H+ as electrolytes, but they still have some problems, which hinder the further development."
Cao explained that multivalent ions as insertion ion electrolytes can significantly improve the EC performance because the number of electrons per multivalent metal ion intercalates into the framework than Li+ or other monovalent ions.
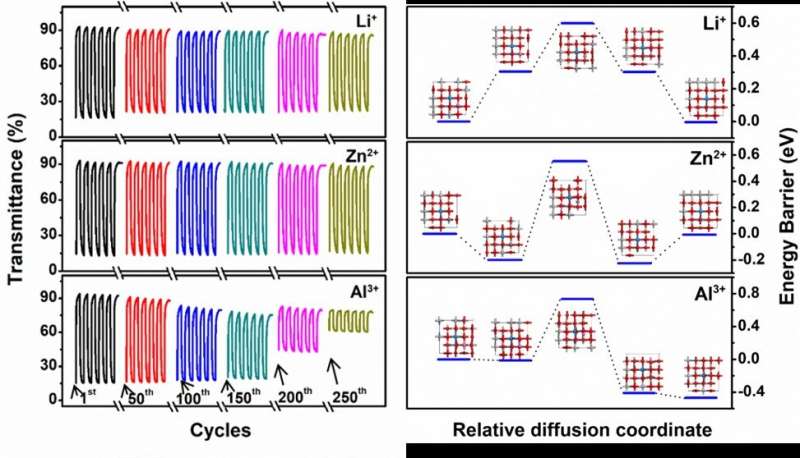
"Zn2+ is regarded as superior to others to trigger the electrochromism due to its simplified preparation process and nontoxicity," Cao said. "Furthermore, the EC device of trivalent Al3+ ion intercalation has attracted extensive attention because of its rich crustal storage, small ion radius, high optical contrast, safety, and reliability.
However, due to the strong electrostatic interaction between multivalent ions and the intercalation framework, there are great difficulties in the intercalation process. According to Cao, so far, the reports on EC performance driven by different valence cation ions mainly focus on the classical tungsten oxide EC materials, and there is a lack of systematic research on other EC materials. Titanium dioxide (TiO2) is a great potential candidate material because of its excellent physical, chemical stability, and acid resistance. However, there is still no report regarding TiO2 for trivalent ions electrochemical cells from the EC community or systematic research on EC performance driven by different valence ions yet.
The challenge is the stronger Coulomb ion lattice interaction of multivalent ions than monovalent ions, Cao said that tungsten doping into TiO2 can reduce the intercalation energy of ions and activate its electrochromic properties. Cao and his team explore the EC properties of anatase W-doped TiO2 NCs in different valence cations (i.e. Li+, Zn2+, and Al3+) by in-situ transmission spectroscopy and electrochemical tests.
"Experimental results and theoretical calculations show that Zn2+ can bring the required fast switching, high contrast, and high stability for EC devices," Cao said. "The research results are of great significance to the basic research in the field of electrochromism and open up a new direction for realizing long-term stable, durable, and fast-switching devices."
More information: Yi Liang et al, Unraveling the Effect of Cation Types on Electrochromic Properties of Titanium Dioxide Nanocrystals, Energy Material Advances (2022). DOI: 10.34133/2022/9878957
Provided by Beijing Institute of Technology Press





















