Developing an eco-friendly ammonia catalyst
A DGIST research team led by Professor Sangaraju Shanmugam, Department of Energy Engineering, developed a catalyst that converts nitric oxide (NO) to ammonia (NH3). This electrochemical technology offers high Faradaic efficiency and low overpotential and produces NH3 in an eco-friendly manner.
NH3 is an important chemical raw material in the fertilizer, textile, and pharmaceutical industries, and it is considered a carbon-free hydrogen carrier with a high energy density. Typically, NH3 is produced using the Haber–Bosch process; however, this process is responsible for approximately 1–2% of global CO2 emissions.
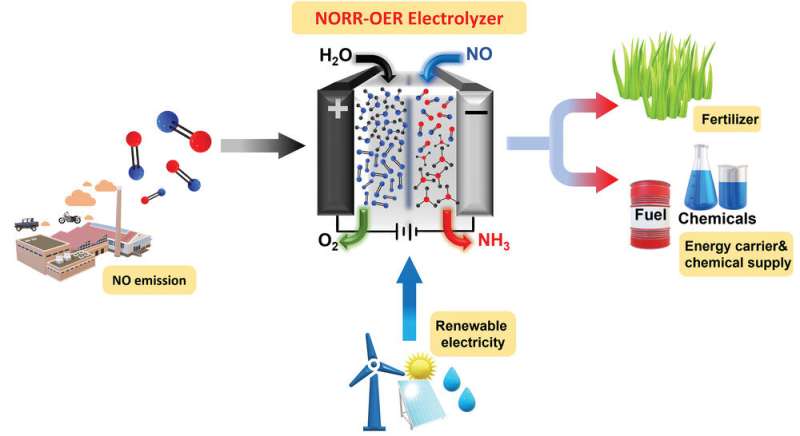
The electrochemical conversion of NO to NH3, an alternative to the Haber–Bosch process, has received considerable attention. This eco-friendly method consumes the air pollutant NO gas to produce NH3. Therefore, this promising approach can replace conventional methods without affecting the environment or emitting CO2.
However, owing to the corrosive nature of NO gas, the morphology of the metal-nanoparticle electrocatalyst degrades during electrosynthesis. Therefore, it is necessary to obtain a catalyst material with high stability that facilitates long-term electrochemical NH3 synthesis.
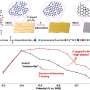
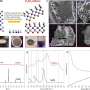
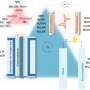
Professor Shanmugam's research team developed a core-shell nickel nanoparticle coated with nitrogen-doped carbon nanostructured electrocatalysts via a simple co-precipitation method for stable NH3 production. This catalyst is stable and efficient and offers a high Faradaic efficiency of 72.3% at a low overpotential (550 mV) in a 100% NO-saturated electrolyte.
Additionally, a solar-to-NH3 efficiency of 1.7% and Faradaic efficiency of more than 50% were achieved using a solar-energy-assisted full-cell PV-NORR electrolyzer.
Professor Shanmugam says that "this research has developed an energy-efficient and eco-friendly technology to synthesize NH3 with zero carbon footprint, and we hope to commercialize this technology to contribute to environmental conservation and sustainability."
The findings of this research were published in Advanced Science.
More information: Sridhar Sethuram Markandaraj et al, Electrochemical Reduction of Nitric Oxide with 1.7% Solar‐to‐Ammonia Efficiency Over Nanostructured Core‐Shell Catalyst at Low Overpotentials, Advanced Science (2022). DOI: 10.1002/advs.202201410
Journal information: Advanced Science
Provided by Daegu Gyeongbuk Institute of Science and Technology