Molecular-level design strategy could hold the key to boosting commercial hydrogen production
Our excessive consumption of fossil fuels is responsible for some of the major societal challenges we are facing, from climate change to pollution. Hydrogen is considered a green alternative to fossil fuels, and alkaline water electrolysis is proving an attractive technology for large-scale commercialization of hydrogen production.
However, current industrial applications of electrocatalytic water splitting are limited by the high overpotential of the oxygen evolution reaction (OER); an important electrochemical reaction in the process. This is especially true when operating at high electrical current densities (500-1000 mA cm-2). In a study published in Green Energy & Environment, a group of researchers from China describe a process they have developed to address this challenge.
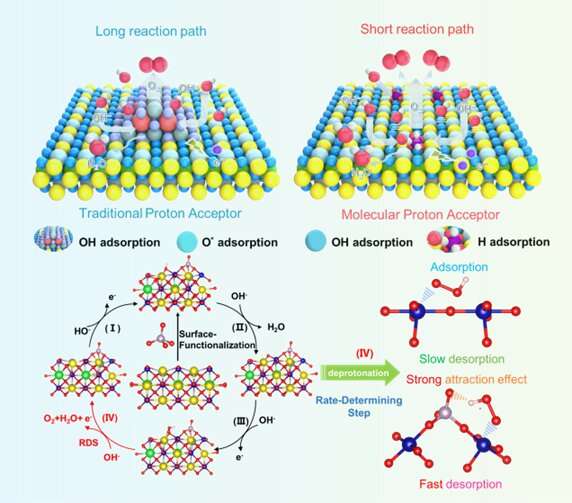
Prof. Yunfei Bu from China's Nanjing University of Information Science and Technology led the research. He explains that "because OER involves four complex proton-electron coordination transfer steps in alkaline media, the level of proton/electron transfer you can achieve is limited. To address that, we constructed a simple and scalable proton acceptor strategy which reduces the size of the proton acceptors to the molecular-level and integrates them into the whole catalyst."
Yaobin Wang, a Ph.D. student at the same university, came up with the new method and, according to co-author Dr. Feng Li, a professor at China's Fudan University, the reason it works so well is that the "molecular-level design increases the direct connection between the surface proton-acceptor and the carrier, and solves the existing problems around a long transfer path, limited interface and loose contact. This results in improved proton transfer kinetics under high current."
The study also evaluated the water electrolysis performance of the catalyst under practical conditions, by using a membrane electrode assembly. According to the researchers, the electrolyzer can achieve a high current density of 500 mA cm-2 or even 1000 mA cm-2 at low overpotential, and the overall Faraday is close to 96%.
Prof. Bu concludes that "this new strategy shows great application prospects in practical water electrolysis devices and high-current industrial applications. In addition, this functional modification at the molecular level has the potential to be extended to more catalytic fields."
More information: Yaobin Wang et al, Molecular-level proton acceptor boosts oxygen evolution catalysis to enable efficient industrial-scale water splitting, Green Energy & Environment (2022). DOI: 10.1016/j.gee.2022.07.001
Provided by KeAi Communications Co., Ltd.