Liquid-interface assisted SERS could see earlier detection of Alzheimer's disease
A new publication from Opto-Electronic Advances discusses label-free trace detection of bio-molecules by liquid-interface assisted surface-enhanced Raman scattering using a microfluidic chip.
Surface-enhanced Raman scattering (SERS) has attracted attention in biotechnology. It is due to its high sensitivity to localized surface plasmon resonance of nanostructured metals. Trace detection of bio-molecules with large molecular weight remains challenging because treating SERS substrate using coupling or cross-linking agents is required. The researchers applied liquid-interface assisted SERS to realize label-free trace detection of bio-molecules. The results suggest it is promising for early-stage diagnosis of virus infection and Alzheimer's.
Surface-enhanced Raman scattering (SERS), based on an optical near-field effect induced by the surface plasmon of noble metal nanoparticles or nanostructures excited by laser radiation, amplifies the Raman signals up to 1014 times compared to regular Raman. Due to its enhanced intensity, the SERS technique continues to attract growing interest in trace-level detection and analysis of biomaterials. It has increased interest in fields such as imaging organelles in a single cell, cancer cell tracking, and biomarker identification.
The SERS technique may be used in the bio-medical field for disease diagnosis at an early stage and also in tumor therapy. Although the enhancement factor of SERS typically ranges from 106– 108 due to the use of novel SERS substrates and methods, single-molecule detection by label-free SERS is impracticable because of SERS-blinking, the origin of this phenomenon being due to the escape of analyte molecules from hotspots. Moreover, bio-molecules, including deoxyribonucleic acid (DNA) and proteins, are difficult to detect directly by SERS. Additional treatments with a SERS substrate are needed to bind the bio-molecules.
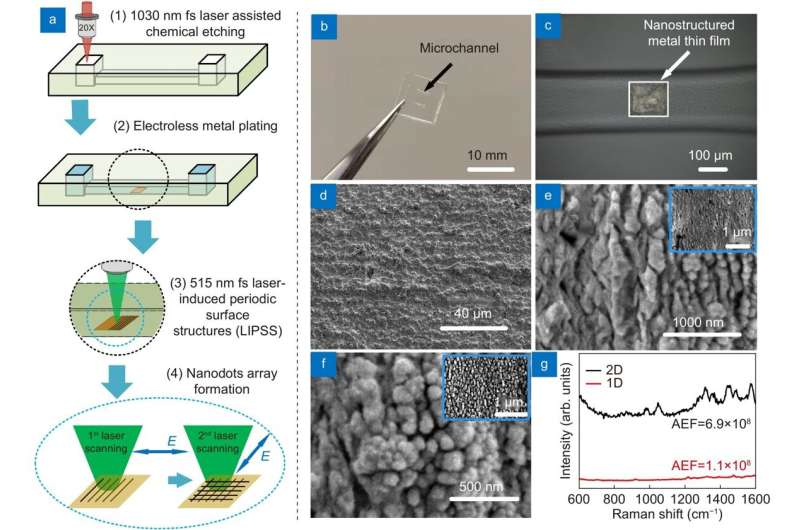
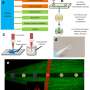
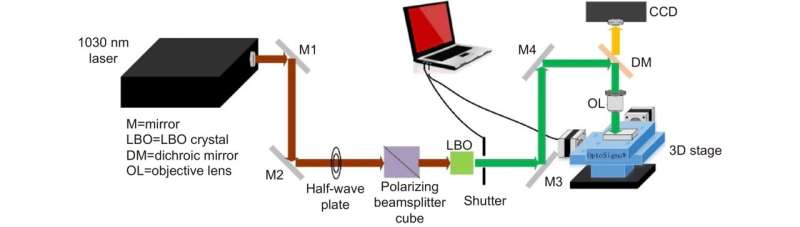
The research team proposed LI-SERS, which achieves a SERS enhancement factor greater than 1014, much higher than the regular SERS method. The microfluidic SERS chip featured an Ag-Cu SERS substrate integrated into an embedded glass microchannel. Hybrid femtosecond (fs) laser processing created the grass microchannel.
The hybrid fs laser processing enables the creation of more complicated 3D structures with enhanced functionalities for biochips, sensors, and microelectronic devices. When the interface between the analyte solution and air on the SERS substrate in the microfluidic channel was irradiated by the Raman excitation laser, the LI-SERS intensity was increased by six orders of magnitude compared with regular SERS. The mechanism of LI-SERS was attributed to the synergetic effect of the Marangoni flow induced by laser irradiation and optical trapping. That laser irradiation would direct the analyte molecules to the hot spots where the collected molecules are trapped by optical force. Consequently, the analyte molecules were immobilized on the SERS substrate with the achievement of strong Raman scattering.
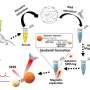
This study demonstrated that the LI-SERS method is applicable for more practical use. It is specifically useful for trace detection of label-free bio-molecules with large molecular masses, including DNA bases, DNA sequences and β-Amyloid (Aβ). Owing to the ultrahigh sensitivity and self-immobilization of LI-SERS, discrimination of DNA bases and DNA sequences with a detection limit of 1 fM was obtained without requiring additional treatments featuring coupling or cross-linking agents. Moreover, the LI-SERS technique can detect label-free Aβ, a biomarker of Alzheimer's disease, at levels below 1 pM, and with a linear correlation between the Raman signal and the Aβ concentration in the range 1 nM–1 pM being achieved. The label-free bio-sensing capability of LI-SERS offers great potential for the early-stage diagnosis of diseases in clinics.
In conclusion, the researchers have provided an overview of the scope of the LI-SERS method for trace detection of bio-molecules in microfluidic SERS chips with particular reference to ultra-trace detection of DNA bases and Aβ. A liquid interface was allowed to form in the microchannel. The Marangoni flow and optical trapping effects induced by LI-SERS demonstrated a detection limit of 1 fM for label-free DNA bases. Notable features of the LI-SERS method, including the ultrahigh sensitivity and versatility associated with collecting and self-immobilizing analyte molecules on the hot spots, will be beneficial for early-stage disease diagnoses such as viral infections and Alzheimer's disease.
More information: Shi Bai et al, Label-free trace detection of bio-molecules by liquid-interface assisted surface-enhanced Raman scattering using a microfluidic chip, Opto-Electronic Advances (2022). DOI: 10.29026/oea.2022.210121
Provided by Compuscript Ltd