Scientists unravel how SUMOylation coordinates endothelial angiogenic signaling
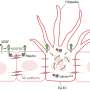
The vascular network is the primary system for transporting nutrients, oxygen and metabolites. Therefore, angiogenesis, the formation of new blood vessels from pre-existing vessels, is of vital importance to the function of organisms. At the core of angiogenesis are endothelial sprouting and lumen formation. The basic fibroblast growth factor (FGF2) has been recognized as the first proangiogenic molecule, and it facilitates angiogenesis by activating FGF receptor 1 (FGFR1) signaling in endothelial cells. However, the precise roles of FGFR and the FGF/FGFR signaling axis in angiogenesis remain obscure.
The research team led by Prof. Yu Luyang and Dr. Qiu Cong at the Zhejiang University College of Life Sciences published an open-access research article titled "FGFR1 SUMOylation coordinates endothelial angiogenic signaling in angiogenesis" in the Proceedings of the National Academy of Sciences on June 21.
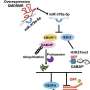
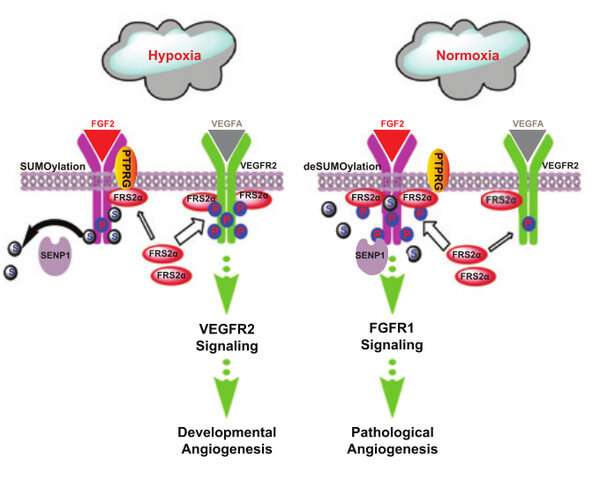
In this study, the researchers identified SENP1-regulated endothelial FGFR1 small ubiquitin-like modifier modification (SUMOylation) at conserved lysines responding to proangiogenic stimuli, while SENP1 functioned as the deSUMOylase. Hypoxia-enhanced FGFR1 SUMOylation restricted the tyrosine kinase activation of FGFR1 by modulating the dimerization of FGFR1 and FGFR1 binding with its phosphatase PTPRG. Consequently, it facilitated the recruitment of FRS2α to VEGFR2 but constrained the additional recruitment of FRS2α to FGFR1, supporting the activation of VEGFA/VEGFR2 signaling in endothelial cells.
Furthermore, the SUMOylation-defective mutation of FGFR1 resulted in exaggerated FGF2/FGFR1 signaling but suppressed VEGFA/VEGFR2 signaling and the angiogenic capabilities of endothelial cells, which were rescued by FRS2α overexpression. Reduced angiogenesis and endothelial sprouting in mice bearing an endothelial-specific, FGFR1 SUMOylation-defective mutant confirmed the functional significance of endothelial FGFR1 SUMOylation in vivo.
These findings identified the reversible SUMOylation of FGFR1 as an intrinsic fine-tuned mechanism in coordinating endothelial angiogenic signaling during neovascularization. Moreover, this study indicated that VEGFA/VEGFR2 signaling primarily operates under hypoxic conditions and that FGF/FGFR1 signaling is more important under normoxic conditions.
More information: Xiaolong Zhu et al, FGFR1 SUMOylation coordinates endothelial angiogenic signaling in angiogenesis, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2202631119
Journal information: Proceedings of the National Academy of Sciences
Provided by Zhejiang University