Researchers use nanotechnology to destroy and prevent relapse of solid tumor cancers
As people across the globe look forward to longer life expectancies, malignant cancers continue to pose threats to human health. The exploration and development of immunotherapy aims to seek new breakthroughs for the treatment of solid tumors.
The successful establishment of anti-tumor immunity requires the activation, expansion and differentiation of antigen-specific lymphocytes. This process largely depends on specific interactions between various T cells and antigen-presenting cells (APCs) in the body. However, existing tumor vaccines, such as neoantigen vaccines and various vector vaccines, all rely on random interactions with APCs in the body. Furthermore, inappropriate interactions may lead to the silencing of other immune responses.
Although immune checkpoint-based immunotherapy has been shown to have great potential, only a small proportion of patients fully respond to this therapy, and the relevant molecular mechanisms need to be further explored. This delivery method is however complex and inefficient.
In a breakthrough development, a team of scientists led by Narat Muzayyin Chair Professor Chen Xiaoyuan from the NUS Yong Loo Lin School of Medicine and Professor Liu Gang from Xiamen University has formulated a novel vaccine which showed high efficacy in the treatment of solid tumors, achieving complete clearance of solid tumors and inducing long-lasting immune memory. This prevents the relapse of tumor growth that the patient originally presented with and provides immunity against similar tumor types. This was proven through the application of this vaccine on melanoma tumor models. Their results are published in Nature Nanotechnology.
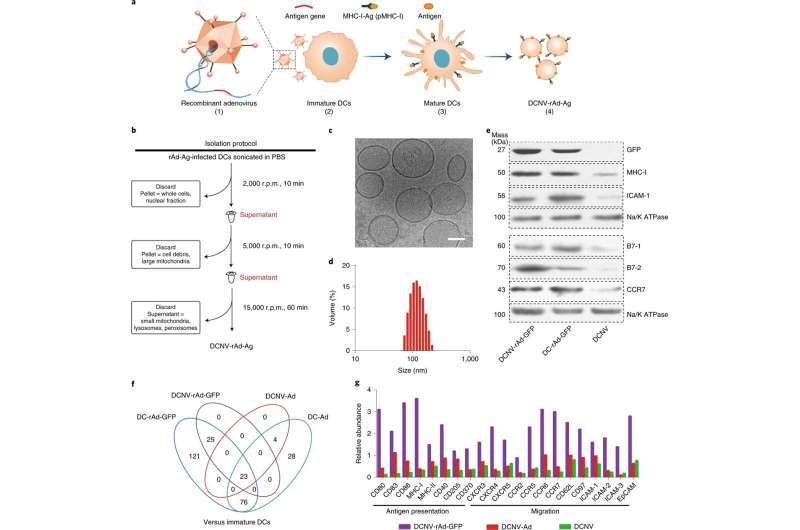
The team was able to engineer a dendritic cell (a type of APC) membrane that was used to naturally stimulate the immune system and activate multi-dimensional anti-tumor immunity. This was done through an antigen self-presentation and immunosuppression reversal nanovesicle vaccine platform, which prompted the team to coin its moniker, ASPIRE.
The ASPIRE vaccine system can quickly elicit appropriate, antigen-specific immune responses in a way that traditional vaccine methods could not. This mode of antigen presentation greatly improves the efficiency of immune activation, which facilitates this novel vaccine's high efficacy relative to other vaccines currently available. In addition, the vaccine can also activate both previously unexposed T cells and exhausted T cells which facilitates ASPIRE's superior anti-tumor immune capabilities.
"We are excited at this platform technology's potential for further application in other diseases as well, such as chronic viral infection, in which T-cell exhaustion often occurs during infection and prevents the optimal viral control," said Prof Chen. "Next, the team hopes to establish a standard operating procedure for scaled synthesis of the vaccine, with proper quality control of the membrane vesicles, for clinical translation," he added.
Speaking independently on the study, Professor Chng Wee Joo, Senior Consultant of the Division of Haematology at the Department of Haematology-Oncology in the National University Cancer Institute, Singapore and myeloma specialist said: "The field of cancer immunotherapy is offering tremendous hope to cancer patients. However, there are some shortcomings with the current technologies. The present innovation from Prof Chen and his colleagues overcome some of these deficiencies and improved the effectiveness and
sustainability of the immune response to these treatments. This will provide a significant advance that will have important impact on patients."
More information: Chao Liu et al, A nanovaccine for antigen self-presentation and immunosuppression reversal as a personalized cancer immunotherapy strategy, Nature Nanotechnology (2022). DOI: 10.1038/s41565-022-01098-0
Journal information: Nature Nanotechnology
Provided by National University of Singapore