The hydroxylation of ASPP2 and other ankyrin repeat domain proteins
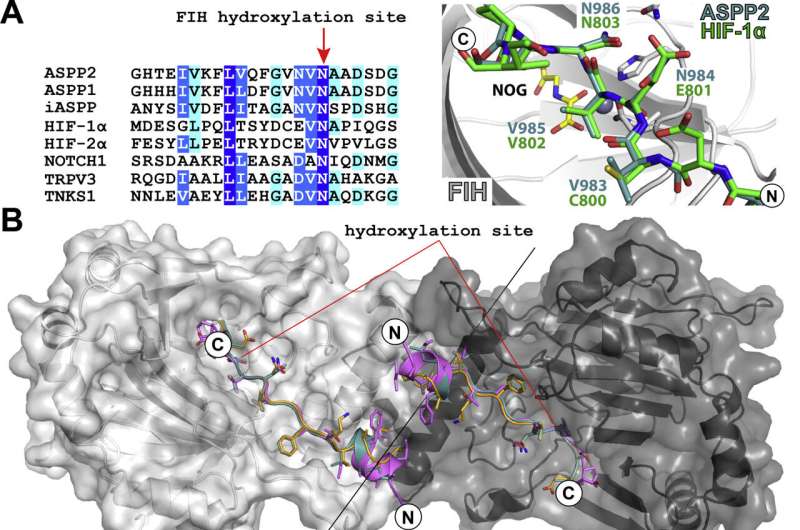
Factor Inhibiting Hypoxia Inducible Factor (FIH) has an important role in the response to low oxygen (hypoxia) in animals. FIH adds a hydroxyl (-OH) group onto the master regulator Hypoxia Inducible Factor (HIF), influencing its ability to activate hundreds of genes that mediate the body's response to hypoxia. It also hydroxylates members of the ankyrin repeat domain protein family, including adding a single hydroxyl group to ASPP2 on asparagine 986.
ASPP2 is involved in several aspects of cancer biology, including the regulation of cell polarity/adhesion and the transcriptional activity of the tumor suppressor protein p53. However, the effect of hydroxylation by FIH on ASPP2's activity is still unclear.
A study led by former Ludwig Oxford researcher Thomas Leissing and his Oxford colleague Christopher Schofield, conducted in collaboration with the labs of Ludwig Oxford Director Xin Lu and Ludwig Member Peter Ratcliffe, investigated the hydroxylation of ASPP2 and other ankyrin repeat domain proteins.
Unexpectedly, in addition to the known site of hydroxylation, the team uncovered the ability of FIH to add hydroxyl groups to both asparagine residues within the "VNVN" motif present in ASPP proteins. Further characterization confirmed this to be a new type of post-translational hydroxylation.
Future work will define the role of this unprecedented modification by FIH in ankyrin repeat domain protein biology and in the response to hypoxia.
This research is published in the Journal of Biological Chemistry.
More information: Thomas M. Leissing et al, Factor inhibiting HIF can catalyse two asparaginyl-hydroxylations in VNVN motifs of ankyrin fold proteins, Journal of Biological Chemistry (2022). DOI: 10.1016/j.jbc.2022.102020
Journal information: Journal of Biological Chemistry
Provided by Ludwig Cancer Research