January 26, 2022 feature
What makes cobalt essential to life?

Cobalt sits in the center of the corrin ring of vitamin B12 and the important cobalamins we derive from it. Perhaps surprisingly, only two of our enzymes bother to use these painfully constructed and meticulously channeled cofactors. Why do our cells go to such great lengths to get a little bit of the cobalt magic, and what catalytic properties might make it so special?
Other uncommon essential metals, like molybdenum, selenium and iodine, are similarly used only sparingly in cells, and yet we retain the ability to completely synthesize all the useful derivatives for these elements. To tame molybdenum, we construct an elaborate molybdopterin cofactor, while to harness iodine, we assemble thyroxine. To incorporate selenium into the few selenoproteins that require it, the elaborate SECIS machinery shuffles the mRNA code to attract a unique tRNA, upon which its cysteine cargo is transformed into selenocysteine. In each of these cases, researchers understand the special properties of the metals involved that make them indispensable.
For example, compared to sulfur, selenium is a better nucleophile that will react with reactive oxygen species faster, but its lack of π-bond character means that it can also be more readily reduced. Selenoproteins like GPX4 (glutathione peroxidase) are correspondingly more resistant to both overoxidation and irreversible inactivation. Similarly, the ineluctable requirement for molybdenum, a two-electron redox compound that can shuttle between the +4/+5 and the +5/+6 redox couples, reflects several not-so-common skills. It can perform diverse and energetically challenging redox reactions; it can act as an electron sink or source at low redox potential; and (along with the much rarer tungsten) can effectively transfer oxygen and sulfur atoms during reactions taking place at low potential.
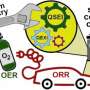
A noteworthy attempt to divine the essential cobalt character was advanced in a recent commentary in PNAS by geochemist extraordinaire Michael Russell. Poised between Fe and Ni in the periodic table, Russell notes that "the element is particularly 'energy-dense' with paired electrons in the outer orbit. Its occurrence as a metal alloy in serpentinites with a variable valence extending from Co+ through to Co4+, its various spin states, and its contrasting conformations render it unique, with untold contributions to be made to electronics, catalysis and the emergence of life. Indeed, Co–Fe cooperation has just been investigated at the opposite end of the redox spectrum—the electrocatalysis of the O2 evolution reaction. Substitutions of Co are either unfeasible, as in metabolism and in some double-atom catalysis, or they lie in the somewhat remote future."
Russell's comments are in response to an earlier article by He et. al. who demonstrated that hydrothermal reduction of bicarbonate into long-chain hydrocarbons (≤24 carbons) is possible through the use of iron and cobalt metals. These findings potentially explain both the abiogenic origin of petroleum, and key events in life's emergence. Since remnants of the porphyrins and corrins that are critical to life can be found amidst petroleum deposits, a critical question becomes: Did life invent these molecules, or did they first use abiotic facsimiles of these molecules and only later evolve the concomitant ability to synthesize them for themselves?
The response to my question from Russell was that, in his opinion, life likely invented corrin-like coordination by way of a four-amino-acid-peptide glycine-glycine-histidine motif capable of entrapping the cobalt atom. Curiously, porphyrins, which house iron or copper in their centers, and chlorins, which do the same with magnesium, must be contracted into corrins to bind cobalt. This specificity seemingly comes despite the near identical atomic radii (around 125 pm) for the contiguous Fe, Co, Ni, Cu elemental lineup. In Russell's view, cobalt (and other transition metals) required at life's emergence were active in deposits of the mineral green rust, also known as fougerite, at alkaline hydrothermal vents. Cobalt corrinoid joined with iron-sulfur clusters form the heart of primitive acetyl coenzyme-A pathways of the acetogens and the methanogens lying at the bottom of our evolutionary tree. This Co(FeS) protein mediates the attachment or detachment of a methyl group to or from carbon monoxide or another entity involved in the biosynthesis of acetyl-CoA.
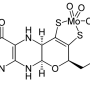
The form of vitamin B12 used by our methylmalonyl-CoA mutase enzyme located in mitochondria for fatty acid and amino acid breakdown is known as adenosylcobalamin (AdoCbl). The other cobalamin-utilizing enzyme, methionine synthase, acts in the cytosol and uses a methylcobalamin cofactor wherein the adenosyl group is replaced by a methyl group. Land plants and fungi neither synthesize or require cobalamin as they lack methylmalonyl-CoA mutase, and have different kind of methionine synthase that doesn't require B12. When these enzymes are not working properly, their precursor molecules can presumably build up to high levels, causing problems like demyelinating disease and pernicious anemia.
While cobalt's thermal stability and high energy density make it an ideal component for the cathodes of lithium batteries, its usefulness to life comes from its many other unique properties, some discovered, and some still yet to be found.
More information: Michael J. Russell, Cobalt: A must-have element for life and livelihood, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2121307119
Journal information: Proceedings of the National Academy of Sciences
© 2022 Science X Network