A missing link in the MHC class divergence puzzle: Class II came first
Which came first, MHC class I or MHC class II? For decades, it has been debated which of these two similar classes came first in evolution. Now, Keiichiro Hashimoto and his group at Fujita Health University, Japan, in collaboration with European research groups, have provided an answer to this question in a new article in PNAS. They discovered an ancient category of MHC molecules, MHC-W, that represents a missing link for explaining how class I molecules evolved from a class II-like origin. Peter Parham, professor at Stanford and the PNAS guest editor who handled the Okamura et al. paper, declares: "In demonstrating that MHC class II evolved prior to MHC class I, which evolved from MHC class II, this landmark study has resolved an important and outstanding puzzle in immunogenetics."
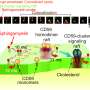
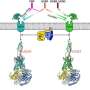
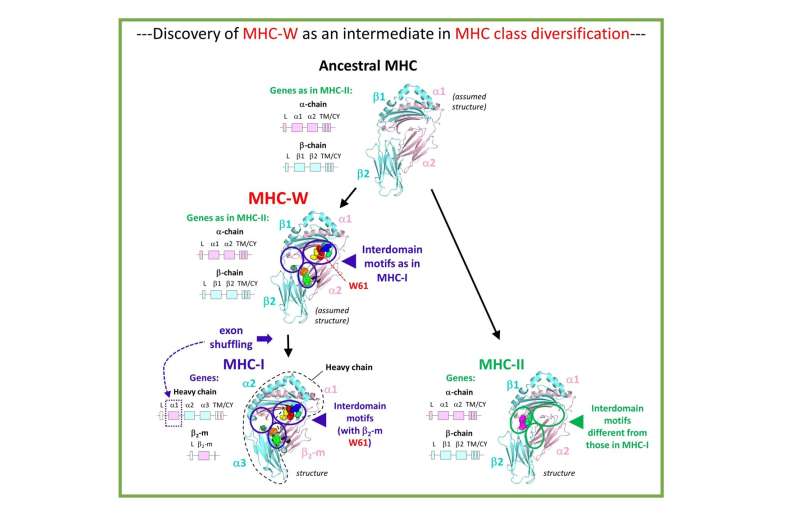
MHC class I and class II molecules are related to each other. Both types possess four extracellular domains of which the two membrane-distal domains form a groove for binding peptides that are presented to T lymphocytes. However, class II molecules are comprised of two transmembrane chains of similar size, the alpha chain possessing the extracellular II-α1 and II-α2 domains and the beta chain possessing the extracellular II-β1 and II-β2 domains. Class I molecules, on the other hand, are comprised of a large transmembrane heavy chain that includes three of the four extracellular domains (I-α1, I-α2, and I-α3) and a free single domain molecule beta-2 microglobulin (β2-m) (Fig. 1). In the structures, the following class I and class II domains correspond with each other: II-α1 with I-α1, II-α2 with β2-m, II-β1 with I-α2, and II-β2 with I-α3. Based on domain similarity levels and considerations of parsimony, Jim Kaufman and co-workers had already proposed in 1984 that in evolution MHC molecules started out as a homodimer followed by gene duplication and differentiation leading to a heterodimer that served as the origin of both extant class I and class II (Fig. 1). However, their conclusion was debated, and until now the question as to which came first was considered unresolved. Importantly, a primitive group of MHC with both class I and class II features, that could shed light on the direction of evolution, had not been found.
By having access to many more MHC sequences from many more species than Jim Kaufman had in 1984, the Hashimoto group could obtain more reliable phylogenetic tree analysis support for the Kaufman model. Most importantly, the Hashimoto group discovered a new category of MHC molecules, "MHC-W," in primitive jawed vertebrates including sharks, ray-finned fishes, lobe-finned fishes, and salamanders, which possess sequences closer related to class I but with similar-sized alpha and beta transmembrane chains as in class II (Fig. 1). This provides final evidence for the Kaufman model as it concludes that MHC molecules with similar-sized alpha and beta transmembrane chains came first in evolution. At the detailed sequence level, it is importantly the interdomain binding motif residues which set MHC-I and MHC-W apart from MHC-II. MHC-W molecules possess residues of the unique critical motifs found in MHC class I complexes for binding of β2-m, including an essential tryptophan (W in single letter code) in the β2-m/Wα2 domain from which MHC-W received its name (Fig. 1). This suggests that the mechanism for binding β2-m evolved before exon shuffling events had created a heavy chain plus β2-m class I situation from an MHC-W ancestor (Fig. 1).
Jim Kaufman, former professor at the University of Cambridge and now professor at the University of Edinburgh, compliments the study: "The research described in Okamura et al. to be published in PNAS is a tour-de-force, with a completely novel and unexpected MHC gene family strongly supported by different kinds of data collected over many years from a range of non-mammalian vertebrate species. The scenario proposed by the lab of Hashimoto is the first advance in many decades for understanding the early evolution of MHC genes."
Apart from providing evidence that in MHC evolution the II-type chain-length organization came first, in the future MHC-W may help with elucidating what is another longstanding enigma in MHC science, namely the mechanism of β2-m function.
More information: Discovery of an ancient MHC category with both class I and class II features, Proceedings of the National Academy of Sciences (2021). DOI: 10.1073/pnas.2108104118
Journal information: Proceedings of the National Academy of Sciences
Provided by Fujita Health University