New dually charged, acid-resistant nanofiltration membranes for ion separation
A research group led by Prof. Wan Yinhua from the Institute of Process Engineering (IPE) of the Chinese Academy of Sciences developed a novel dually charged acid-resistant nanofiltration (NF) membrane for efficient ion separation.
The study was published in the Journal of Membrane Science Letters on Nov. 22.
NF, as an environmentally friendly pressure-driven separation technology, performs well in divalent ions and organics removal. It has been widely applied in wastewater treatment.
Unfortunately, for the most prevalent polyamide thin-film composite membrane prepared by interfacial polymerization, the skin layer usually suffers from structure deterioration in extreme low pH conditions. Moreover, commonly used acid resistant NF membranes tend to have low permselectivity due to the uneven and thick separation layer structure.
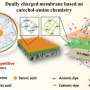
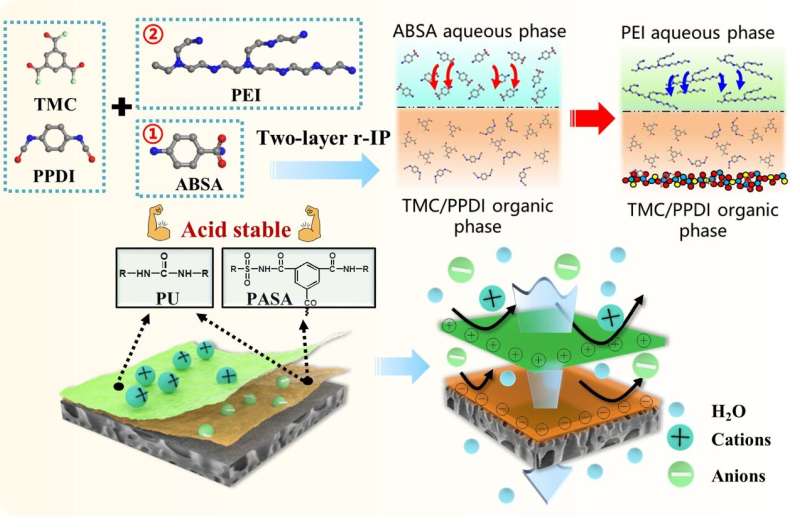
The researchers prepared a dually charged acid-resistant NF membrane with remarkable cations and anions rejections based on a facile two-layer reverse interfacial polymerization process.
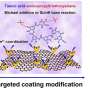
In this strategy, mixed organic monomers of trimesoyl chloride (TMC) and 1,4-phenylene diisocyanate (PPDI) were used to react with 3-aminobenzenesulfonamide (ABSA) to prepare a negatively charged hybrid interlayer of poly(amide-sulfonamide) (PASA) and polyurea. This was followed by the reaction of TMC/PPDI with polyethyleneimine to construct a hybrid positively charged layer of polyamide and polyurea on the interlayer surface.
"The acid stable PASA and highly cross-linked polyurea effectively prevent the direct attack of acid. Importantly, the polyurea with high hydrogen bond density also leads to an enhanced size sieving effect," said Prof. Wan.
In addition, by changing the monomer usage order during interfacial polymerization, the opposite diffusion direction of the monomers would layer the charge distribution to attenuate the charge neutralization effect.
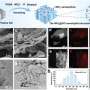
Interestingly, due to the low hydration energy and high hydrogen bond density of the top layer, the permeate flux of the resultant membrane is much higher when filtrating salt solution than pure water. Consequently, the membrane with well-arranged dually charged layer shows high electrostatic exclusion to both anions and cations (Na2SO4: 97.7%, MgCl2: 93.0%), as well as higher permeability than commercial acid resistant membrane.
Moreover, this dually charged membrane shows excellent heavy metal removal and long-term stability in strong acid.
"This work not only offers new insights into structure regulation of NF membrane, but also provides a novel strategy to fabricate acid-resistant membrane with higher permeate flux," said Prof. Luo Jianquan from IPE, the corresponding author of the study. "Such NF membrane is promising to be applied in fast heavy metal removal and other ionic separations such as water softening and desalination."
More information: Yang Cao et al, A novel acid resistant thin-film composite nanofiltration membrane with polyurea enhanced dually charged separation layer, Journal of Membrane Science Letters (2021). DOI: 10.1016/j.memlet.2021.100002
Provided by Chinese Academy of Sciences