Pore-mouth catalysis boosting the formation of iso-paraffins from syngas over bifunctional catalysts
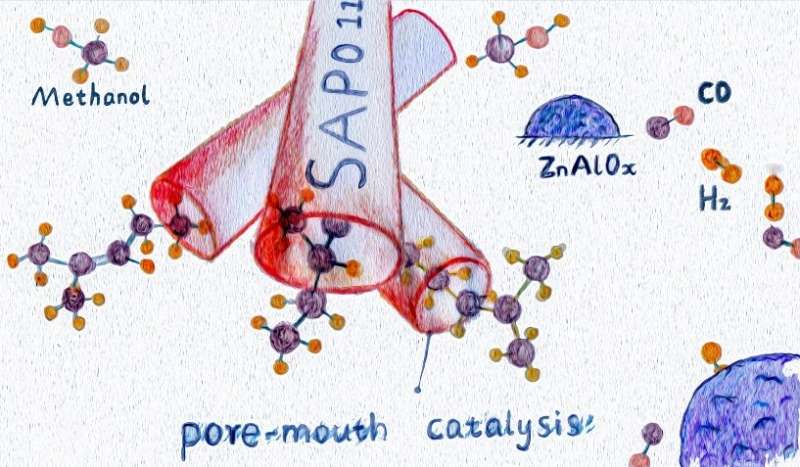
The bifunctional catalysts consisting of spinel ZnAlOx and SAPO-11 zeolite can directly convert syngas (a mixture of H2 and CO) into high-quality gasoline. The selectivity of C5–C11 gasoline-range hydrocarbons can reach 79% with a high content of iso-paraffins. The formation of iso-paraffins over ZnAlOx/SAPO-11 catalyst follows a pore-mouth catalysis mechanism, which means the isomerization of linear hydrocarbons can only take place near the pore mouth region of zeolite channels.
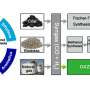
Catalytic transformation of syngas (a mixture of H2 and CO), an important platform for the utilization of non-oil carbon resources including coal, natural gas, CO2, and even carbon-containing waste, into hydrocarbon products has drawn much research attention in recent years. Over conventional Fischer-Tropsch (FT) catalysts, the formation of C–C chains on metallic sites is uncontrollable and the distribution of hydrocarbons is governed by the Anderson-Schulz-Flory (ASF) distribution. According to the ASF theory, the formation of branched hydrocarbons with high octane number is forbidden and the maximum selectivity for linear gasoline-range hydrocarbons (C5–C11) is limited to 48%. To increase the selectivity of C5–C11, a new type of bifunctional catalyst consisting of metal oxide and zeolite has been discovered. These catalysts separated CO activation (into methanol or ketene) and C–C coupling on two different sites, thus enabling the precise synthesis of C5–C11 with exceptionally high selectivity. However, up to now, the formation mechanism of iso-paraffins and the comparison with the conventional FT/zeolite catalyst have not been investigated.
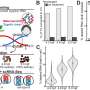
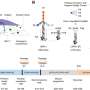
Recently, a research team led by Prof. Ye Wang from Xiamen University has designed a bifunctional catalyst consisting of spinel ZnAlOx and one-dimensional SAPO-11 zeolite with ten-membered-ring channels. It was found that an appropriate micropore size of SAPO-11 zeolite, high pressure, moderate temperature, and a close distance between bifunctional components can promote the formation of the C5−C11 gasoline-range hydrocarbons. Under optimized conditions, the selectivity of C5−C11 range hydrocarbons can reach 79% at a CO conversion of 24%, which is much higher than the conventional FT process. Interestingly, ZnAlOx/SAPO-11 catalyst can preferentially catalyze the formation of iso-paraffins, with an iso-paraffins to n-paraffins ratio in the C5−C11 range as high as 13. Besides, the majority of hydrocarbon molecules are mono-branched. Considering the micropore of SAPO-11 can only accommodate linear hydrocarbon molecules, it is proposed that the formation of mono-branched isomers follows a pore-mouth catalysis mechanism, which means the isomerization of linear hydrocarbons can only take place near the pore mouth region of zeolite channels. Compared with di-branched isomers, the mono-branched isomers have a lower tendency for cracking by acid sites, thus can survive in harsh reaction conditions. This might be one of the reasons that the product selectivities of ZnAlOx/SAPO-11 were very stable during the course of the reaction. This work presents the first example of pore-mouth catalysis in C1 chemistry, and preliminarily reveals the formation mechanism of iso-paraffins over the emerging bifunctional oxide-zeolite catalysts. The results were published in Chinese Journal of Catalysis.
More information: Mengheng Wang et al, Pore-mouth catalysis boosting the formation of iso-paraffins from syngas over bifunctional catalysts, Chinese Journal of Catalysis (2021). DOI: 10.1016/S1872-2067(20)63770-6
Provided by Chinese Academy of Sciences