Researchers harness higher order protein catenation for the development of artificial antibodies
Chemical topology is a unique dimension for protein engineering. Over the past few years, the discovery of topological non-trivial proteins in nature has already revealed their many potential functional benefits, such as enhanced thermal/mechanical/chemical stability. Engineering the chemical topology of proteins thus holds the promise of engineering therapeutically relevant proteins and industrial enzymes.
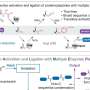
The assembly-reaction synergy has been established as an effective strategy for the synthesis of topological proteins. Two types of genetically encoded chemical tools are mandated: One for selective chain entanglement into certain geometry, the other for site-specific covalent ligation. To date, only a few motifs have been identified in the former category and found suitable for the synthesis of topological proteins; more effective entwined protein motifs are urgently needed. Recently, Prof. Zhang Wen-Bin's research group has designed a highly efficient entwined protein heterodimer via engineering the p53dim homodimer, and using it to synthesize complexed protein higher order [n]catenanes, which showed potential applications as artificial antibodies.
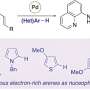
The homodimer p53dim is an entangled homodimeric mutant of the tetramerization domain of the tumor suppressor protein p53. In this work, by changing pairs of neutral hydrophilic residues on its dimeric interface to either both positively or both negatively charged residues, the electrostatic repulsion between similarly charged residues prevents homodimer formation while the electrostatic attraction between oppositely charged residues promotes heterodimer formation, thus giving rise to a pair of entwined protein heterodimers: X+(T5R/Q7R) and X−(T5E/Q7E) (T: threonine, Q: glutamine, R: arginine, E: glutamic acid). The engineered p53dim heterodimer enables the entanglement of different protein chains selectively. Its combination with orthogonal genetically encoded protein cyclization tools, such as split intein, allows the cellular synthesis of protein heterocatenanes with various proteins of interests (POIs).
During co-expression of the primary protein ring scaffold containing two or three tandem X− motifs and the secondary ring containing one X+, their assembly followed by mutually orthogonal ring closure reactions (split intein and SpyTag-SpyCatcher chemistry) promotes the effective formation of protein [3] or [4]catenane. The higher order protein catenane scaffold tolerates the insertion of various POIs, such as human epidermal growth factor receptor 2-specific affibody (AffiHER2) or sfGFP, providing a good candidate to design multivalent functional protein systems. Incorporating AffiHER2 onto the [n]catenane scaffold produces artificial antibody (termed [n]catbody) with improved binding affinity (~8 folds for [4]catbody), prolonged serum half-life (~10 folds), and good tumor accumulation.
This research successfully realized the synthesis of complexed topological proteins via expanding the toolkits of protein entangling motifs, promoting the study of their structure-property relationships and leading to advanced protein therapeutics.
This work, titled "Higher Order Protein Catenation Leads to an Artificial Antibody with Enhanced Affinity and In Vivo Stability," was recently published in the Journal of the American Chemical Society. The first author is Dr. Wu Wen-Hao and the corresponding authors are Professors Zhang Wen-Bin and Wei Wei.
More information: Wen-Hao Wu et al, Higher Order Protein Catenation Leads to an Artificial Antibody with Enhanced Affinity and In Vivo Stability, Journal of the American Chemical Society (2021). DOI: 10.1021/jacs.1c06169
Journal information: Journal of the American Chemical Society
Provided by Peking University