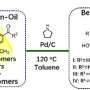
Redoxdivergent strategy for construction of (Dihydro)thiophenes with dimethyl sulfoxide
(Dihydro)thiophenes, among the most common five-membered heterocycles, are widespread in a large number of natural products, functional materials, and biologically active compounds.
Sulfide sources are usually employed to prepare thiophene compounds through the formation of two new C–S bonds. However, the substrates employed are highly functionalized precursors, leading to limited scope and functional group compatibility.
Recently, a research team led by Prof. Chen Qing'an from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences developed the redoxdivergent construction of (dihydro)thiophenes with dimethyl sulfoxide (DMSO) as both oxidant and sulfur donor.
This study was published in Angewandte Chemie International Edition on August 30.
The researchers employed readily available allylic alcohols as starting materials and DMSO as mild oxidant to offer derivatives of (dihydro)thiophenes efficiently. They found that the manipulation of the selectivity could be governed by the dosage of DMSO and HBr.
In addition, they demonstrated that this redoxdivergent strategy could realize programmable and concise synthesis of tetraarylthiophenes, bioactive DuP 697 and its regioisomers. It may serve as a general platform for achieving synthetically and medicinally useful five-numbered sulfur-containing heterocycles.
More information: Heng Liu et al, Redoxdivergent Construction of (Dihydro)thiophenes with DMSO, Angewandte Chemie International Edition (2021). DOI: 10.1002/anie.202109026
Journal information: Angewandte Chemie International Edition
Provided by Chinese Academy of Sciences