Response to DNA damage: Dual role of extramitochondrial cytochrome C
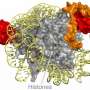
![Domain architecture and N-end structure of ANP32B. (A) Schematic representation of ANP32B domain organization. The N-end structured domain of ANP32B is colored in blue, whereas the histone chaperone LCAR is represented in yellow. The four LRRs are colored in red. ANP32B Nuclear Localization Signal (NLS) is represented in orange and the histone chaperone Nuclear Export Signal (NES) is marked on the image. ANP32B Thr244 residue is represented in grey. (B) Ribbon representation of ANP32B (PDB: 2ELL [12]) N-terminal domain following the color scheme described in A. ANP32B structure is rotated 90° around the horizontal axes in each view. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.) Response to DNA damage: Dual role of extramitochondrial cytochrome C](https://scx1.b-cdn.net/csz/news/800a/2021/response-to-dna-damage.jpg)
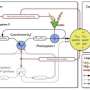
Living beings are continuously exposed to harmful agents, both exogenous (ultraviolet radiation, polluting gases, etc.) and endogenous (secondary products of cellular metabolism) that can affect DNA integrity. That's why cells are endowed with a series of molecular mechanisms whose purpose is to identify and signpost possible damage to the genetic material for speedy repair. These mechanisms are precisely regulated because they are key to cell survival. In extreme situations of massive and irreparable damage, cells enter a phase of controlled dismantling called "programmed cell death." Among the events that take place during this process is the massive delivery to the cytoplasm of a mitochondrial protein called cytochrome C. Under homeostatic conditions, this protein plays a role in energy metabolism within the mitochondria. However, in situations of irreparable damage, it directs the controlled and orchestrated destruction of the cell from the cytoplasm.
The authors of this paper suggest that extramitochondrial cytochrome C plays a dual role in response to cell damage. In the initial stages of damage, a limited amount of cytochrome C is able to reach the cell nucleus without accumulating in the cytoplasm and, therefore, without triggering programmed cell death. Once in the nucleus, cytochrome C interacts with the protein ANP32B, a histone chaperone that helps maintain DNA structure and inhibits the activity of the enzyme (phosphatase) PP2A, a major promoter of DNA damage repair. Thus, cytochrome C "hijacks" ANP32B as a means of activating PP2A and facilitating DNA repair. When damage to the genetic material cannot be repaired by the cellular mechanisms, cytochrome C "floods" both the cytoplasm and the nucleus, leading to the irremediable death of the cell. "What's the point of continuing to build a house that's going to be demolished?"
More information: Francisco Rivero-Rodríguez et al, Inhibition of the PP2A activity by the histone chaperone ANP32B is long-range allosterically regulated by respiratory cytochrome c, Redox Biology (2021). DOI: 10.1016/j.redox.2021.101967
Provided by University of Seville