Helium, a little atom for big physics
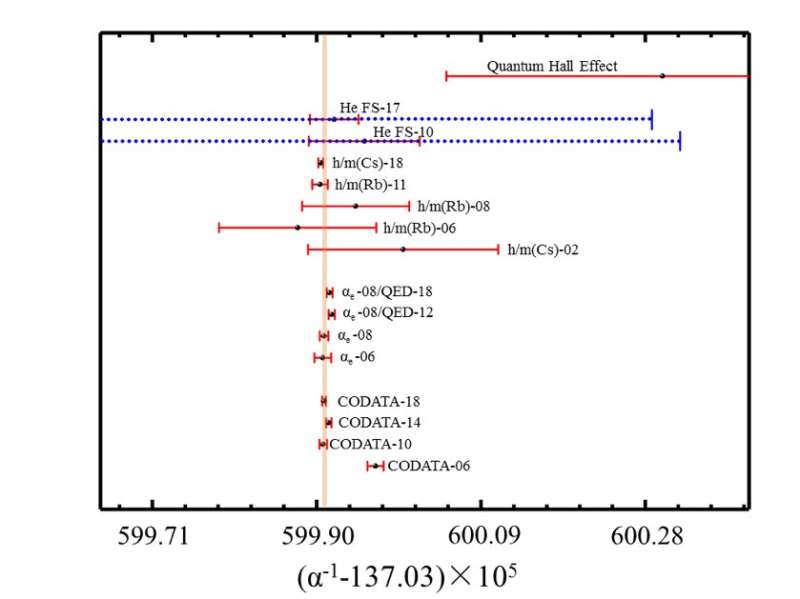
Helium atom precision measurements and calculations have a history of nearly a century. In the 1960s, theorists discovered that the fine-structure split (23P0-23P2) of the 23P energy level of helium is the best atomic system for measuring the fine structure constant α (approximately 1/137), which is the key parameter in the Quantum Electrodynamics (QED) theory. QED is the basic theory describing the quantum properties of electromagnetic interactions. It covers almost all physical systems from microscopic particles to macroscopic solids, and is currently the most accurate theory in physics. Such a measurement of α from precision spectroscopy of helium, compared with values determined from totally different methods, presents a perfect test of the consistency of physics. After 50 years of hard work, theorists have develoed different approaches to calculate the QED correction of helium to the 7th power series of α.
Experimental precision measurements of helium atoms have been carried out in many international research institutions. Recent experimental progresses obtained in several groups worldwide are introduced, including the 2S-2P transition frequency of He-4 and the 23P0-23P2 fine structure interval determined by the authors' research group, which are the most accurate results to date.
At present, the accuracy of calculated results of helium is limited by the very complicated QED correction of the 8th order of α. On the one hand, it may be developed through theoretical development, and on the other hand, it may be explored through precision measurements of other helium-like ions. This will be an extremely strict test of QED.
In addition, precision measurement of helium also has a broad impact to various important studies.
Spectroscopy of the helium atom has been applied to determine the radius of helium nuclei. At present, there is still a significant deviation between the measured results of the difference between the nuclear radius of helium-3 and helium-4. The reason for this deviation has not been explained, and the solution of this problem will provide an important reference to solve the 'puzzle of proton radius.'
The polarizability of helium atoms can be accurately calculated and the refractive index of helium gas can be derived. Since the refractive index of a gas can be precisely measured by optical methods, this becomes a metrology method for optically determining the density (pressure) of gases. Related technical methods are under development at NIST in the United States and at PTB in Germany, and the authors' research team has also undertaken related research.
More information: Y R Sun et al, Precision Spectroscopy of Atomic Helium, National Science Review (2020). DOI: 10.1093/nsr/nwaa216
Provided by Science China Press