Dynamic full-field optical coherence tomography: 3-D live-imaging of retinal organoids
Optical coherence tomography offers astounding opportunities to image the complex structure of living tissue but lacks functional information. We present dynamic full-field optical coherence tomography as a technique to noninvasively image living human induced pluripotent stem cell (hiPSC)-derived retinal organoids. Colored images with an endogenous contrast linked to organelle motility are generated, with submicrometer spatial resolution and millisecond temporal resolution, creating a way to identify specific cell types in living tissue via their dynamic profile.
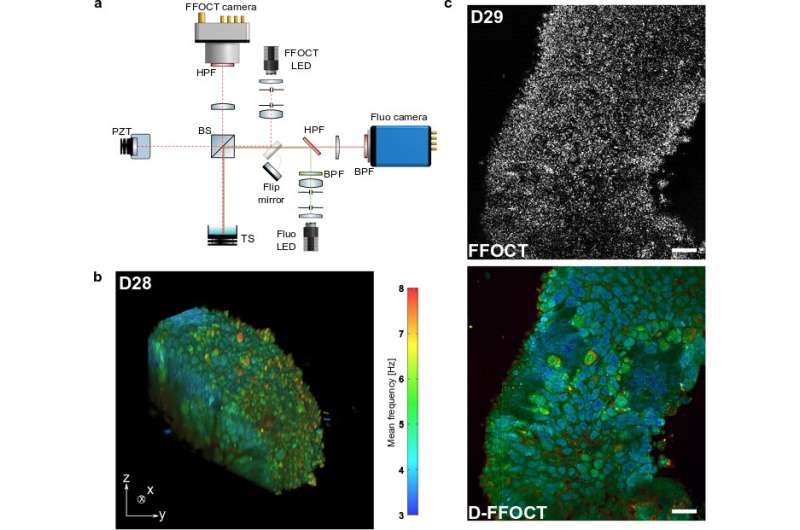
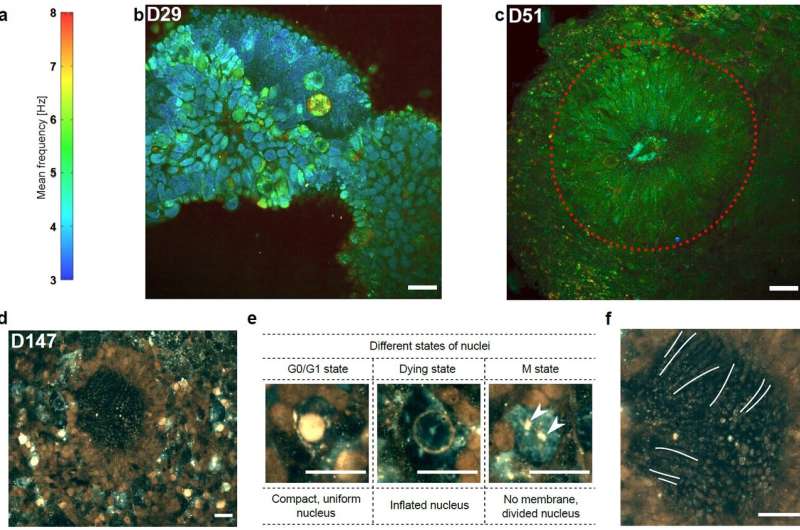
Current modalities for imaging living tissues and 3-D cell cultures are invasive, slow or lacking in spatial resolution. Dynamic full-field optical coherence tomography (D-FFOCT) is a label-free, non-invasive, quantitative technique allying high spatial and temporal resolutions. This technique relies on low coherence interferometry to amplify the phase and amplitude fluctuations, created by moving scattering structures inside biological samples, yielding a motility contrast. D-FFOCT opens up the possibility of following the development of complex 3-D multicellular structures, such as retinal organoids.
In a new paper by Jules Scholler, Kassandra Groux, et al., published in Light: Science & Applications, a team of optics experts (Institut Langevin, Paris, France) led by Dr. Kate Grieve from the Quinze-Vingts National Eye Hospital (Paris, France), in collaboration with cell biologists (Institut de la Vision, Paris, France), have developed and applied a new imaging modality for the imaging of in-development retinal organoids.
These scientists summarize the operational principle of their microscope:
"We use the interferometric amplification of a full field optical coherence tomography device and study the fluctuation of the interferometric signal to quantitatively construct tomographic volumes with a metabolic contrast. Owing to our high sensitivity, we are able to reconstruct highly contrasted images of almost transparent samples without using any exogenous labels."
"Owing to the full field configuration and the high sensitivity, our method is faster and requires much lower illumination intensity than nonlinear microscopy techniques that can damage the sample irreversibly. This allows us to study the development of the same sample over periods of several weeks" they added.
"D-FFOCT will have many potential applications for in vitro living tissue including disease modeling, cancer screening, and drug screening," the scientists predict.
More information: Jules Scholler et al, Dynamic full-field optical coherence tomography: 3D live-imaging of retinal organoids, Light: Science & Applications (2020). DOI: 10.1038/s41377-020-00375-8
Journal information: Light: Science & Applications
Provided by Chinese Academy of Sciences