Research powers longer lasting rechargeable batteries
With the continuous improvement of electronics, the development of high-energy power supplies has become a key link in the future development of science and technology. However, the shortage of lithium resources and the difficulty of recycling have become important factors in limiting their development.
Non-lithium based rechargeable batteries with an inexhaustible supply of raw materials, such as sodium ion batteries (SIBs), have attracted widespread attention in recent years. As the critical determinant for the energy output of SIBs, the development of cathodes has made exciting progress, including layered materials, polyanions and Prussian blue analogs (PBAs), etc.
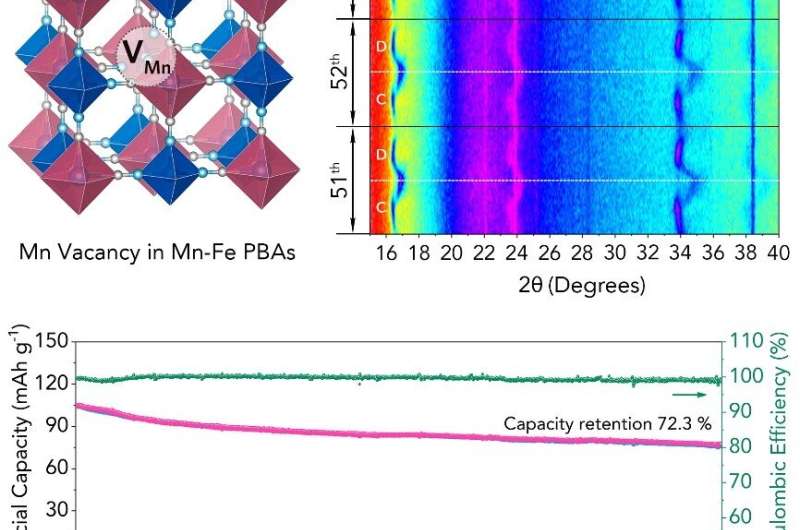
Among these cathodes, Mn-based Prussian blue analogues (Mn-Fe PBAs, Na2Mn[Fe(CN)6]) represent one of the most promising cathode materials for SIBs due to their higher theoretical capacity and adaptive volume variation. However, Mn-Fe PBAs suffer from poor cycling reversibility and capacity retention during the phase transition from cubic to tetragonal phase, which is related to the large structural deformation of Mn-N6 octahedra caused by Jahn-Teller distortion.
Previous efforts to suppress the large structural deformation mainly focused on optimized phase structure or partial atomic replacement, but these methods could not maintain a stable cycle while maintaining a high capacity, which is necessary for the practical application of batteries.
In the study that was published in Chem, the researchers have developed a controllable strategy to create unconventional cation Mn vacancies (VMn) on Mn-Fe PBAs by using a strong chelating agent, ethylenediaminet etraacetic acid disodium (Na2EDTA). The VMn in Mn-Fe PBAs could restrain the movement of Mn-N bonds and thereby mitigate the Jahn-Teller distortion of Mn-N6 octahedra, leading to highly reversible phase transitions of NMF as well as an outstanding long-term cycling stability and capacity retention (refer to image).
Due to the strong chelating effect of EDTA2-, Mn2+ and EDTA2- chelated to form a highly stable six-coordinate octahedron that not only greatly slows down the release rate of Mn2+ as well as the formation rate of EDTA-NMF, but also removes the Mn atoms from the crystal lattice. As the reaction proceeds, the strong coordination of Na2EDTA would continue to etch the NMF and create more VMn on the surface.
The VMn in Mn-Fe PBAs could act as the first barrier to prevent the structural damage during battery cycling. As a result, the Mn-Fe PBAs exhibited an outstanding long-term cycling stability and capacity retention for both half-cell (72.3% after 2700 cycles at 0.5 A g-1) and full-cell (75.5% after 550 cycles at 0.1 A g-1).
"Considering the facile synthesis and great diversity of PBAs, this work not only promotes creative synthetic methodologies for controllable defect or vacancy engineering, but also opens unlimited avenues to explore the relationship between structure, vacancies and electrochemical performances in materials beyond PBAs," said lead author Associate Professor Yang Hui Ying from SUTD.
More information: Yang Shang et al, Unconventional Mn Vacancies in Mn–Fe Prussian Blue Analogs: Suppressing Jahn-Teller Distortion for Ultrastable Sodium Storage, Chem (2020). DOI: 10.1016/j.chempr.2020.05.004
Journal information: Chem
Provided by Singapore University of Technology and Design