Hotel Ruthenium: how hydrogen checks in but never leaves
How does hydrogen form blisters in ruthenium mirrors for extreme UV (EUV) lithography machines? An M2i research project by Chidozie Onwudinanti and colleagues at DIFFER, Eindhoven University of Technology and University of Twente explains the blistering process: a layer of tin contamination acts as a valve that lets hydrogen into the underlying ruthenium, but blocks it from leaving again, writes the team in the journal Physical Chemistry Chemical Physics.
How does hydrogen form blisters in ruthenium mirrors for extreme UV (EUV) lithography machines? An M2i research project by Chidozie Onwudinanti and colleagues at DIFFER, Eindhoven University of Technology and University of Twente explains the blistering process: a layer of tin contamination acts as a valve that lets hydrogen into the underlying ruthenium, but blocks it from leaving again, writes the team in the journal Physical Chemistry Chemical Physics.
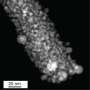
Extreme ultraviolet lithography (EUV) machines are quite extraordinary pieces of technology, which sometimes run into the sorts of problems that arise when pushing the limits of what is physically possible. One such problem is the damage suffered by the mirrors in the machines. EUV light is absorbed by all solid materials, and by air as well, so the light in these machines is focused and directed by mirrors in near-vacuum. The light comes from a tin plasma, the mirrors topped with ruthenium direct the light, and hydrogen gas acts as a buffer and cleaning agent for the mirrors. This otherwise perfect dance is ruined by the formation of blisters, high-pressure pockets of hydrogen underneath the ruthenium cap, when tin debris lands on the mirrors.
Ph.D. candidate and main author Chidozie Onwudinanti: "Our earlier work had established that hydrogen and tin stick readily to the ruthenium surface, and that tin proximity aids hydrogen penetration into the ruthenium. However, hydrogen solubility in ruthenium is low. So we faced the question: how do so many hydrogen atoms get into, and through the ruthenium layer to form blisters?"
Valve for hydrogen
Transition-state calculations of hydrogen diffusion paths from the near-surface region of ruthenium revealed the mechanism: hydrogen can move deeper into the metal, but it cannot leave through the surface because the surface is saturated with hydrogen and tin. Onwudinanti: "In other words, having reduced the barrier for hydrogen entry into the subsurface, tin makes it more difficult for the hydrogen to leave through the top of the film. We found a similar surface-blocking effect was found in a number of different experiments with hydrogen permeation through metals, also in applications for nuclear fusion."
"What we have shown is how tin acts as a valve of sorts, letting the hydrogen pass through the surface, but mostly in one direction. In truth, the hydrogen does leave the ruthenium; it simply leaves at the wrong side of the film. In future work we intend to look more closely at the process of tin deposition on the ruthenium surface, and how hydrogen plays into it, and we will apply other computational techniques to the problem. We aim to build up a more complete picture about the key factors and their effects on the rate of blistering."
More information: Chidozie Onwudinanti et al. Hydrogen diffusion out of ruthenium—an ab initio study of the role of adsorbates, Physical Chemistry Chemical Physics (2020). DOI: 10.1039/D0CP00448K
Journal information: Physical Chemistry Chemical Physics