Chromium speciation in marine carbonates and implications on atmospheric oxygenation
The oxidation of Earth's early atmosphere and ocean played an important role in the evolution of life. Reconstructing the paleo-redox conditions is crucial for the understanding of the coevolution of life and environment. The Cr isotopic composition in sedimentary rocks have been increasingly used as an emerging paleo-redox indicator. It is largely based on the assumption that when the atmospheric oxygen level is relatively high, oxidation of Cr(III) in terrestrial rocks will result in soluble Cr(VI).
This process can lead to positively fractionated Cr isotopic composition of Cr(VI), which will ultimately be transported to the ocean and preserved in sedimentary rocks. Carbonates are widely distributed sedimentary rocks and are an important geological archive. Previous studies suggested that Cr(VI) in seawater can be directly incorporated into carbonate crystal lattice, and marine non-skeletal carbonate could directly record the Cr isotopic composition of the contemporaneous seawater. However, there is hitherto no direct evidence for the presence of Cr(VI) in sedimentary carbonates.
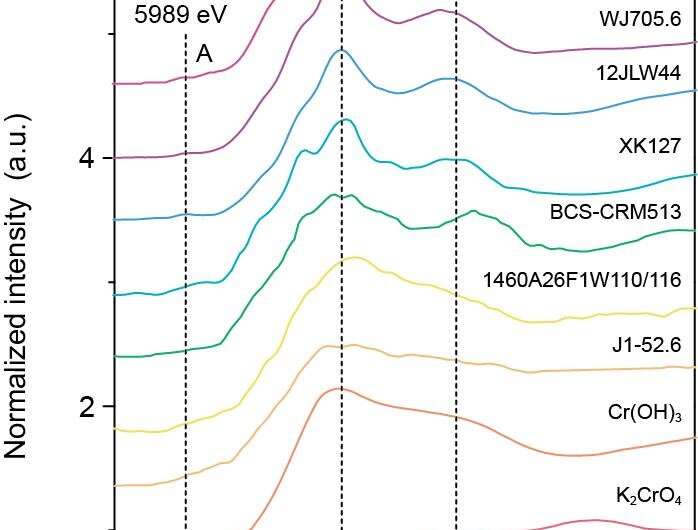
To address whether Cr(VI) is present in sedimentary carbonates, Ziyao Fang and colleagues applied synchrotron based X-ray absorption near edge structure (XANES) spectra to geochemical studies. They examined the Cr valence states and Cr isotopic compositions of sedimentary carbonates formed in different geological periods. Their results showed that Cr(III) dominates in all sedimentary marine carbonates, even in those which have positively fractionated Cr isotopic compositions. This is in apparent contrast with the previous assumption.
Fang and colleagues proposed two possible mechanisms for the absence of Cr(VI) in carbonates. One is that Cr(VI) in seawater might have been reduced by microbes or porewater during carbonate precipitation or early diagenesis. In this case, the reduction of Cr(VI) is likely not quantitative in the relatively oxic carbonate deposition environment, and as a result, the isotopic composition of carbonate would be lighter than that of contemporaneous seawater. The other possible mechanism is that carbonates preferentially direct uptake of Cr(III) in seawater, especially those formed in early geological times when the oxygen level is widely regarded as low. Trivalent Cr can come from non-redox Cr cycling, and recent studies suggest that dissolution and adsorption of Cr(III) can also cause positive Cr isotopic fractionation. Therefore, the slightly positively fractionated Cr isotopic values recorded in some sedimentary carbonates do not necessarily correspond to the presence of oxidative Cr(III) weathering as previously suggested.
More information: Ziyao Fang et al, Absence of hexavalent chromium in marine carbonates: implications for chromium isotopes as paleoenvironment proxy, National Science Review (2020). DOI: 10.1093/nsr/nwaa090
Provided by Science China Press



















