Invisible X-rays turn blue
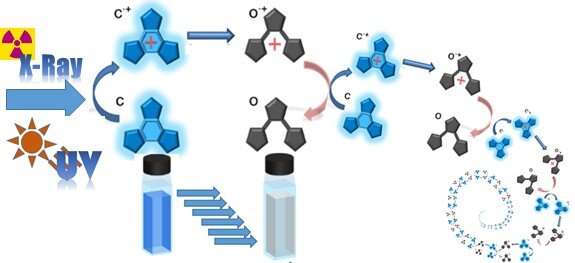
A new reaction system can detect X-rays at the highest sensitivity ever recorded by using organic molecules. The system, developed by researchers at Nara Institute of Science and Technology (NAIST), Ikoma, Japan; and Centre National de la Recherche Scientfique (CNRS), Toulouse, France, involves the cycloreversion of terarylene, causing the molecule to switch reversibly between colorless and blue isoforms in the presence or absence of X-rays. With detection at safe doses, this reaction system is expected to detect even the faintest X-rays levels considered dangerous.
Photoreactive materials convert light input into chemical output and are standard in semiconductor and 3-D printing technologies. Some of these materials are also used in eye-protection, e.g., sunglasses that can reduce UV exposure by changing the lens color. Similarly, workers at risk of X-ray radiation exposure are required to wear monitoring badges that indicate dangerous levels through changes in photoreactive materials. However, NAIST Professor Tsuyoshi Kawai stresses that these badges do not completely eliminate the risk.
"Current materials for wearable detectors are sensitive to about 1 Gy. Ideally, safety management systems want about one hundred times more sensitivity," he says.
Kawai is an expert at increasing the photoconversion efficiency of photoreactive molecules, having focused his attention primarily on terarylenes, organic molecules with which his research team has consistently achieved exceptionally high reaction efficiencies.
"We have steadily improved the number of molecules that can undergo photoconversion in response to one photon. It was one to one in 2011 and today it becomes 33 molecules per one photon," he says.
To increase the quantum yield of terarylenes is to maximize the number of changes that can be induced by a single photon. They have selected terarylenes because of their reversibility, meaning that the molecule can be converted back to the starting blue isoform upon exposure to ultraviolet light allowing for the system to be reset for repeated use.
Indeed, the color change is one of several reasons he believes organic molecules are preferable when considering X-ray detectors.
"Photochromic organic detectors can report X-rays through easily observed color changes and are recyclable and easy to process," he says.
The key modification to the terarylene molecules was the addition of a phenyl group to only one of the molecules two phenylthiophene groups, which allowed for reversible photoconversion between two isoforms. The result was a sensitivity of up to 0.3 Gy, making it more than 1000 times more sensitive than current commercial systems. Notably, 0.3 Gy is considered a safe exposure level, suggesting that no dangerous level will go undetected.
Photoconversion reactions like photosynthesis or neural stimulation in response to light in our eyes occurs at less than 100% efficiency (less than one molecule reacts to one photon). The system designed by the researchers, however, could achieve 3300% (33 molecules per photon), showing the potential of organic molecules in artificial systems.
"I think this is the highest efficiency ever reported for photoconversion with an organic molecule," notes Kawai.
More information: Ryosuke Asato et al, Photosynergetic amplification of radiation input: from efficient UV induced cycloreversion to sensitive X-ray detection, Chemical Science (2020). DOI: 10.1039/C9SC05380H
Journal information: Chemical Science
Provided by Nara Institute of Science and Technology