Artificial 'inclusion bodies' created for controlled drug release
Precision medicine is becoming increasingly important, creating more efficient personalised therapies for each patient and innovative pharmacological developments. In the oncology field, for example, researchers are developing different approaches aimed at directed and controlled drug release systems, thereby diminishing toxicity to the organism.
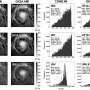
In this sense, researchers at the CIBER's Bioengineering, Biomaterials and Nanomedicine sector (CIBER-BBN), the Institute of Biotechnology and Biomedicine of the Universitat Autònoma de Barcelona (IBB-UAB) and the Hospital Sant Pau Research Institute have developed a new type of protein biomaterial capable of a sustained release of therapeutic proteins administered subcutaneously in lab animals.
"These structures, measuring a few micrometres in diameter, contain functional proteins that are released similarly to how human hormones are released by the endocrine system," states Antonio Villaverde, researcher at the UAB and the CIBER-BBN and one of the study's coordinators.
The study is the result of a stable scientific collaboration between Antonio Villaverde's group and the group led by Ramon Mangues at the Hospital Sant Pau Research Institute. It also included the involvement of the Institute for Biological and Technological Research of the National University of Cordoba-CONICET in Argentina.
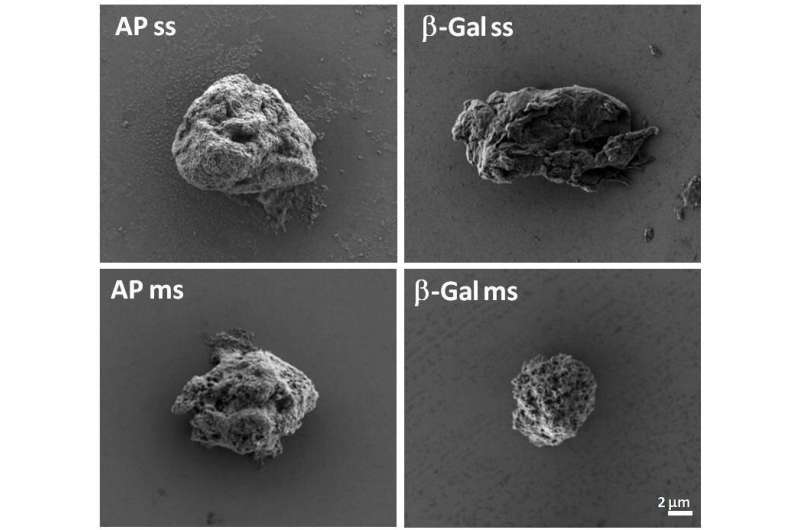
Dr. Mangues, also a researcher at the CIBER-BBN and co-author of the paper, explains that "the new biomaterial imitates a bacterial product commonly found in biotechnological processes known as 'inclusion bodies,' that are pharmacologically of interest, and which in this artificial version offer a wide array of therapeutic possibilities for the oncological field and any other clinical sector in which a sustained release is needed."
Researchers used as models enzymes common to biotechnology and a nanostructured bacterial toxin directed at human colorectal cancer metastatic cells, which have been tested on animal models. "In this way, we were able to generate as many immovable catalysts a new anti-tumour drug with prolonged action," the leading authors of the study explain.
Enormous Clinical Potencial
The artificial protein granules developed here, which had previously been proposed as "nanopills" (therapeutic pills at nanoscopic scale), imitate the action of bacterial inclusion bodies and have enormous clinical potential for vaccines and controlled-release drug delivery systems.
"We have seen that natural inclusion bodies, administered as drugs, can produce undesired immune system responses due to the inevitable contamination of the bacterial materials," researchers state. However, in this new study, the development of artificial inclusion bodies with a secretion capacity "prevents many of the regulatory problems associated with the potential development of bacterial 'nanopills,' and offers a transverse platform through which to obtain functional components for cosmetic and clinical uses," they add.
This study suggests that artificial inclusion bodies can become a new category of exploitable biomaterials to be used in biotechnological applications, due to the facility with which they are manufactured and the foresight of future clinical applications.
More information: Julieta M. Sánchez et al, Artificial Inclusion Bodies for Clinical Development, Advanced Science (2019). DOI: 10.1002/advs.201902420
Provided by Autonomous University of Barcelona