Elusive molecule, first in Universe, detected in space
In the beginning, more than 13 billion years ago, the Universe was an undifferentiated soup of three simple, single-atom elements.
Stars would not form for another 100 million years.
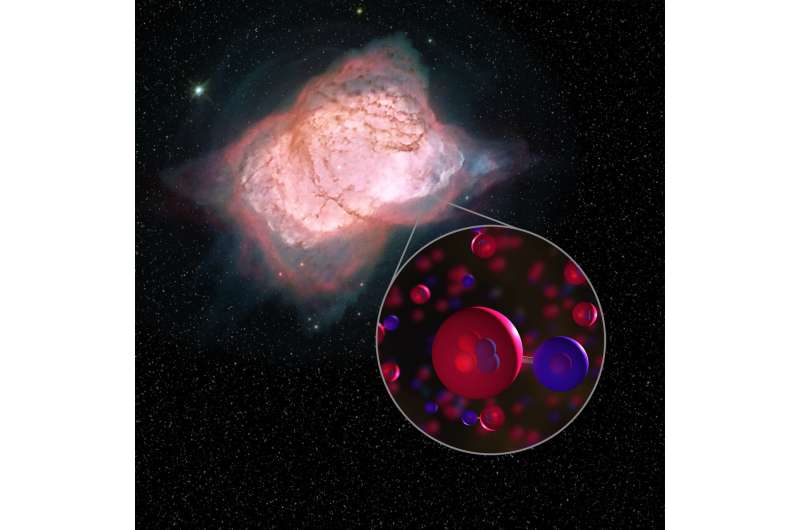
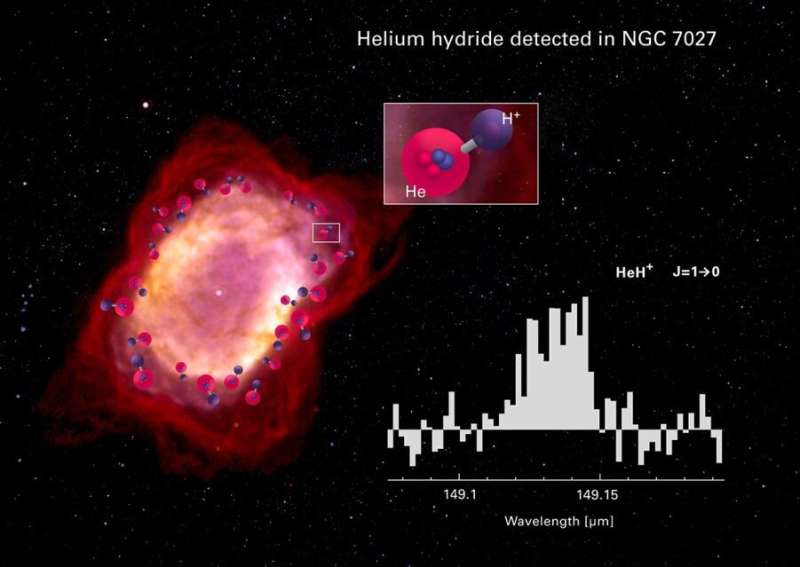
But within 100,000 years of the Big Bang, the very first molecule emerged, an improbable marriage of helium and hydrogen known as a helium hydride ion, or HeH+.
"It was the beginning of chemistry," said David Neufeld, a professor at John's Hopkins University and co-author of a study published Wednesday detailing how—after a multi-decade search—scientists finally detected the elusive molecule in space.
"The formation of HeH+ was the first step on a path of increasing complexity in the Universe," as momentous a shift as the one from single-cell to multicellular life on Earth, he told AFP.
Theoretical models had long since convinced astrophysicists that HeH+ came first, followed—in a precise order—by a parade of other increasingly complex and heavy molecules.
HeH+ had also been studied in the laboratory, as early as 1925.
But detected HeH+ in its natural habitat had remained beyond their grasp.
"The lack of definitive evidence of its very existence in interstellar space has been a dilemma for astronomy for a long time," said lead author Rolf Gusten, a scientist at the Max Planck Institute for Radioastronomy in Bonn.
Researchers knew where to look.
Already in the 1970s, models suggested that HeH+ should exist in significant quantities in the glowing gases ejected by dying Sun-like stars, which created conditions similar to those found in the early Universe.
A fragile molecule
The problem was that the electromagnetic waves given off by the molecule were in a range—far-infrared—cancelled out by Earth's atmosphere, and thus undetectable from the ground.
So NASA and the German Aerospace Center joined forces to create an airborne observatory with three main components: a massive 2.7-metre telescope, an infrared spectrometer, and a Boeing 747—with a window-like square cut away from it fuselage—big enough to carry them.
From a cruising altitude of nearly 14,000 metres (45,000 feet), the Stratospheric Observatory for Infrared Astronomy, or SOFIA, avoided 85 percent of the atmospheric "noise" of ground-based telescopes.
Data from a series of three flights in May 2016 contained the molecular evidence scientists had long sought, interlaced in the planetary nebula NGC 7027 some 3,000 light years away.
"The discovery of HeH+ is a dramatic and beautiful demonstration of Nature's tendency to form molecules," said Neufeld.
In this case, it did so despite inauspicious circumstances.
Even though temperatures in the young Universe fell rapidly after the Big Bang, they were still in the neighbourhood of 4,000 degrees Celsius, a hostile environment for molecular bonding.
Moreover, helium—a "noble" gas—"has a very low proclivity for form molecules," Neufeld explained.
It's union with ionised hydrogen was fragile, and did not persist for very long, replaced by progressively more robust and complex molecular bonds.
Heavier elements such as carbon, oxygen and nitrogen—and the many molecules they gave rise to—were formed later still by the nuclear reactions that power stars.
More information: Rolf Güsten et al. Astrophysical detection of the helium hydride ion HeH+, Nature (2019). DOI: 10.1038/s41586-019-1090-x
Journal information: Nature
© 2019 AFP