Spontaneous spin polarization demonstrated in a two-dimensional material
Physicists from the University of Basel have demonstrated spin alignment of free electrons within a two-dimensional material. Writing in the latest edition of Nature Nanotechnology, they described their observation of spontaneous spin polarization, which cannot occur in ideal two-dimensional materials according to a well-known theorem from the 1960s.
Two-dimensional materials are the subject of numerous studies. As they are only a few atomic layers thick, they have different physical properties from their three-dimensional equivalents. Graphene, a single layer of carbon atoms arranged in a honeycomb pattern, promises to deliver entirely new applications thanks to its notable electronic properties and is the best-known example of this group of innovative materials.
Professor Richard Warburton from the Department of Physics and the Swiss Nanoscience Institute of the University of Basel leads a group studying two-dimensional materials that are also suitable for optical applications. One particularly promising candidate is a single monolayer of molybdenum disulfide (MoS2), as this material has a band gap—unlike graphene—and can therefore emit light when excited.
All in the same direction
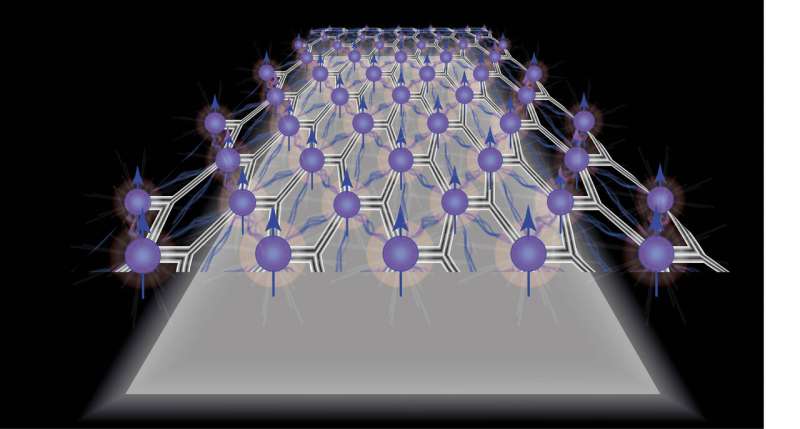
Now, in the latest analyses of two-dimensional molybdenum disulfide layers, doctoral students Jonas Roch and Nadine Leisgang have made a very surprising discovery. They filled the MoS2 layer with free electrons and then exposed it to a weak magnetic field.
This caused the intrinsic angular momentum (spin) of all free electrons to point in the same direction, and the spin could be "switched" to the other direction by reversing the magnetic field. Known as "spontaneous spin polarization," this phenomenon came as a complete surprise because a theorem from the 1960s rules out spontaneous spin polarization in an ideal two-dimensional material.
"Where does the spin polarization come from? The electrons are interacting with one another, and molybdenum disulfide also exhibits a very weak spin-orbit coupling. These two factors presumably have a massive influence on the system," explains Jonas Roch. The formulation of the 1966 theorem had assumed an absence of spin-orbit interaction.
"The results show how exciting experimental physics can be, and how we're constantly learning new things about two-dimensional materials," says Richard Warburton.
More information: Spin-polarized electrons in monolayer MoS2, Nature Nanotechnology, DOI: 10.1038/s41565-019-0397-y , www.nature.com/articles/s41565-019-0397-y
Journal information: Nature Nanotechnology
Provided by University of Basel