A water-splitting catalyst unlike any other

Electricity can be generated by renewable sources such as sunlight and wind, then used to split water, which makes hydrogen as a fuel for emerging energy devices such as fuel cells. Because hydrogen is a clean fuel, researchers are putting a lot of effort into developing water-splitting catalysts, which are essential for the reaction's energy efficiency.
The focus is mostly on the so-called oxygen evolution reaction (OER), which is arguably the most challenging process in water splitting. After many years of intense research, nickel-iron oxide is now established as the go-to catalyst for OER in alkaline conditions due to its high activity and earth-abundant composition, and also because it has the highest activity per reaction site among all metal oxides.
About three years ago, scientists with the lab of Xile Hu at EPFL discovered another catalyst that was significantly more active than nickel-iron oxide, even though it had a similar composition. It is robust, easy to synthesize, and open to industrial applications.
The discovery was led by Fang Song, a postdoc in Hu's group who has since joined the faculty at Shanghai Jiaotong University in China. Recognizing its technological potential, Hu, Song, and their colleague Elitsa Petkucheva began testing the catalyst in a proof-of-concept project. The catalyst enabled an efficient electrolyzer that could work under industrial conditions while requiring 200 mV less voltage.
But the new catalyst was also unconventional in terms of chemistry. "We didn't have a clue why the catalyst would be so active," says Hu. So his team turned to the group of Clemence Corminboeuf at EPFL for help. Working with her postdoc, Michael Busch, Corminboeuf used density functional theory (DFT) computations to search for possible theoretical explanations. DFT is a computational, quantum mechanical method that models and studies the structure of many-body systems, e.g. atoms, and molecules.
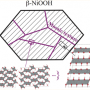
The result was radical: The high activity of the new catalyst originates from a cooperative action of two phase-separated components of iron and nickel oxides, which overcame a previously identified limitation of conventional metal oxides where the reaction occurred locally on only a single metal site. They called it the bifunctional mechanism.
While the DFT-derived mechanism was hypothetical, it guided experimental studies on the activity and properties of the catalyst with Benedikt Lassalle-Kaiser at Synchrotron SOLEIL in France. Using X-ray absorption spectroscopy (XAS), the work uncovered evidence of two phase-separated iron and nickel oxides in the catalyst. But because catalysts can undergo compositional and structural changes during catalysis, it became necessary to study the catalyst in operation with XAS.
In a comprehensive operando XAS study, Chen and his graduate student, Chia-Shuo Hsu, revealed a unique structure of the catalyst—it is made of nanoclusters of γ-FeOOH covalently linked to a γ-NiOOH support, which makes it an iron-nickel oxide catalyst, as opposed to the conventional nickel-iron oxide. Although not a direct proof, this structure is compatible with the DFT-proposed bifunctional mechanism.
"This is a truly interdisciplinary study involving many fruitful collaborations," says Hu. "The fundamental studies not only provide insights into the structure and activity of this unconventional catalyst, but also lead to a thought-provoking mechanistic hypothesis."
More information: Fang Song et al, An Unconventional Iron Nickel Catalyst for the Oxygen Evolution Reaction, ACS Central Science (2019). DOI: 10.1021/acscentsci.9b00053
Journal information: ACS Central Science
Provided by Ecole Polytechnique Federale de Lausanne