Scientists design fast, reversible bio-inspired catalysts
For wind and sun power to become renewable energy mainstays, the energy they produce intermittently needs to be stored and retrieved efficiently. And that requires storing solar energy in chemical bonds until the energy is needed. To be energy efficient, and thereby cost-effective, there is a great need for reversible catalysts, chemical agents that rapidly form and break chemical bonds in either direction.
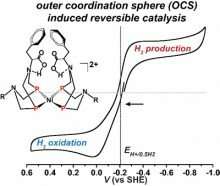
Now, using a bio-inspired design that mimics nature's catalysts-enzymes, researchers at Pacific Northwest National Laboratory (PNNL) have designed a rarely seen reversible synthetic catalyst. Their research, a collaboration among PNNL postdoctoral chemist Arnab Dutta and staff chemists Wendy Shaw and Aaron Appel, was reviewed in a Nature Reviews Chemistry article titled, "Designing electrochemically reversible H2 oxidation and production catalysts." The article describes three generations of catalysts that achieved reversible catalysis, a hallmark of efficient enzymatic behavior. It reviews not only their research but also progress on design features needed to develop reversible catalysts for hydrogen oxidation and production using natural metalloenzymes as models.
"Developing reversible and fast catalysts is considered the 'holy grail' in catalysis research," said Dutta, now an assistant professor at the Indian Institute for Technology, Gandhinagar. "It provides the most efficient tool for interconversion of chemical species with almost zero energy waste."
With their reversibility and their highly efficient and intricately assembled structures, enzymes provide an attractive model for synthetic catalysts. The study describes the design of energy-efficient electrocatalysts that mediate forward and reverse reactions at high rates with minimal energy loss. Specifically, they developed a series of molecular nickel catalysts and found by properly positioning enzyme-inspired amino acids they could maintain fast rates for oxidation of hydrogen.
The critical insight involved designing the active site structure inside an intricately connected protein-inspired scaffold.
"The structured protein scaffold is crucial for the amazing efficiency of enzymatic activity as it orchestrates the thermodynamic and kinetic aspects-the energy requirements and speed-of the catalytic cycle," said Dutta.
The novel design diverges from typical catalyst design, which often focuses on the metal core and the immediate environment around the metal, with little attention paid to regions beyond the active site. To develop highly active and efficient synthetic catalysts using non-precious metal complexes, the extended structure needs to be used and carefully designed, according to the research team.
The research team is now working on extending these results, which were accomplished in solution, to the dry fuel-cell conditions necessary for industrial application. They are working with collaborators on a prototype bio-fuel cell that would incorporate the results achieved in this research.
More information: Arnab Dutta et al. Designing electrochemically reversible H2 oxidation and production catalysts, Nature Reviews Chemistry (2018). DOI: 10.1038/s41570-018-0032-8
Provided by Environmental Molecular Sciences Laboratory