Research suggests life thrived on Earth 3.5 billion years ago
Three and a half billion years ago, Earth hosted life, but was it barely surviving, or thriving? A new study carried out by a multi-institutional team with leadership including the Earth-Life Science Institute (ELSI) of Tokyo Institute of Technology (Tokyo Tech) provides new answers to this question. Microbial metabolism is recorded in billions of years of sulfur isotope ratios that agree with this study's predictions, suggesting that life flourished in the ancient oceans. Using this data, scientists can more deeply link the geochemical record with cellular states and ecology.
Scientists want to know how long life has existed on Earth. If it has been around for almost as long as the planet, this suggests it is easy for life to originate and should therefore be common in the universe. If it takes a long time to originate, this suggests there were very special conditions that had to occur. Dinosaurs, whose bones are presented in museums around the world, were preceded by billions of years by microbes. While microbes have left some physical evidence of their presence in the ancient geological record, they do not fossilize well, thus scientists use other methods for understanding whether life was present in the geological record.
Presently, the oldest evidence of microbial life on Earth comes to us in the form of stable isotopes. The chemical elements charted on the periodic table are defined by the number of protons in their nuclei. For example, hydrogen atoms have one proton, helium atoms have two, carbon atoms contain six. In addition to protons, most atomic nuclei also contain neutrons, which are about as heavy as protons, but which don't bear an electric charge. Atoms that contain the same number of protons, but variable numbers of neutrons, are known as isotopes. While many isotopes are radioactive and thus decay into other elements, some do not undergo such reactions; these are known as "stable" isotopes. For example, the stable isotopes of carbon include carbon 12 (written as 12C for short, with 6 protons and 6 neutrons) and carbon 13 (13C, with 6 protons and 7 neutrons).

All living things, including humans, "eat and excrete." That is to say, they take in food and expel waste. Microbes often eat simple compounds made available by the environment. For example, some are able to take in carbon dioxide (CO2) as a carbon source to build their own cells. Naturally occurring CO2 has a fairly constant ratio of 12C to 13C. However, 12CO2 is about 2 percent lighter than 13CO2, so 12CO2 molecules diffuse and react slightly faster, and thus the microbes themselves become "isotopically light," containing more 12C than 13C, and when they die and leave their remains in the fossil record, their stable isotopic signature remains, and is measurable. The isotopic composition, or "signature," of such processes can be very specific to the microbes that produce them.
Besides carbon, there are other chemical elements essential for living things. For example, sulfur, with 16 protons, has three naturally abundant stable isotopes, 32S (with 16 neutrons), 33S (with 17 neutrons) and 34S (with 18 neutrons). Sulfur isotope patterns left behind by microbes thus record the history of biological metabolism based on sulfur-containing compounds back to around 3.5 billion years ago.
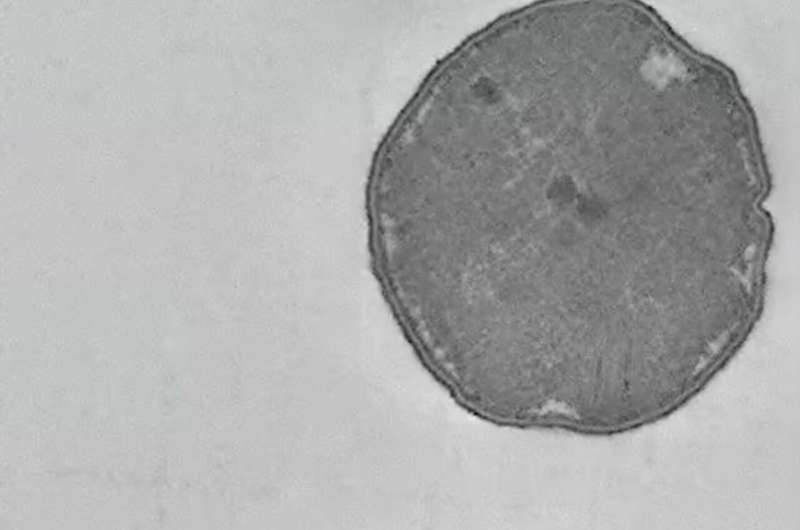
Hundreds of previous studies have examined wide variations in ancient and contemporary sulfur isotope ratios resulting from sulfate (a naturally occurring sulfur compound bonded to four oxygen atoms) metabolism. Many microbes are able to use sulfate as a fuel, and in the process excrete sulfide, another sulfur compound (Figure 1). The sulfide "waste" of ancient microbial metabolism is then stored in the geological record, and its isotope ratios can be measured by analyzing minerals such as the FeS2 mineral pyrite shown in Figure 2.
This new study reveals a primary biological control step in microbial sulfur metabolism, and clarifies which cellular states lead to which types of sulfur isotope fractionation. This allows scientists to link metabolism to isotopes: By knowing how metabolism changes stable isotope ratios, scientists can predict the isotopic signature organisms should leave behind.

This study provides some of the first information regarding how robustly ancient life was metabolizing. Microbial sulfate metabolism is recorded in over 3 billion years of sulfur isotope ratios that are in line with this study's predictions, which suggest life was in fact thriving in the ancient oceans. This work opens up a new field of research, which ELSI Associate Professor Shawn McGlynn calls "evolutionary and isotopic enzymology." Using this type of data, scientists can now proceed to other elements, such as carbon and nitrogen, and more completely link the geochemical record with cellular states and ecology via an understanding of enzyme evolution and Earth history.
More information: Min Sub Sim et al, Role of APS reductase in biogeochemical sulfur isotope fractionation, Nature Communications (2018). DOI: 10.1038/s41467-018-07878-4
Journal information: Nature Communications
Provided by Tokyo Institute of Technology