Molecular inhibition gets cells on the move
Researchers at Osaka University show how the mutual inhibition of two molecules results in their localization at opposite ends of cells, acting as a trigger for the formation of appendages at one cell end that makes directional cell movement possible
To respond to changes in their environment, individual cells, including some of those that compose complex organisms, need to move. This can be accomplished by propelling themselves using projections called pseudopodia. Although this system is present within many different species, the mechanisms that control it and enable cells to move in a particular direction have not been completely clarified.
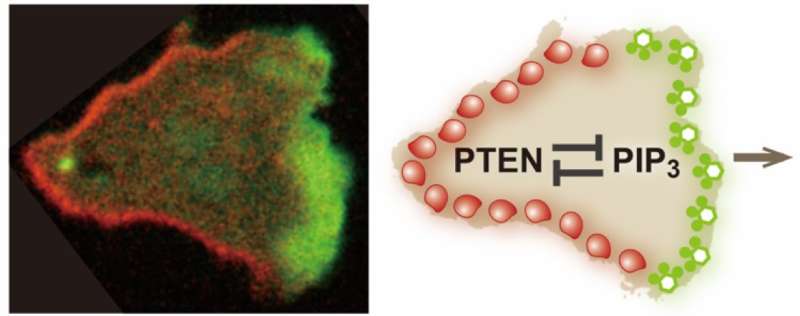
In recent work published in the journal Nature Communications, researchers at Osaka University have revealed how cells in an organism known as a slime mold establish the polarized distribution of two molecules in their outer membranes. The localization of these two molecules exclusively at different ends of a cell leads to the assembly of machinery for pseudopodium formation only at one cell end. This ensures a unidirectional force from pseudopodia used to propel cells, enabling directed cell movement.
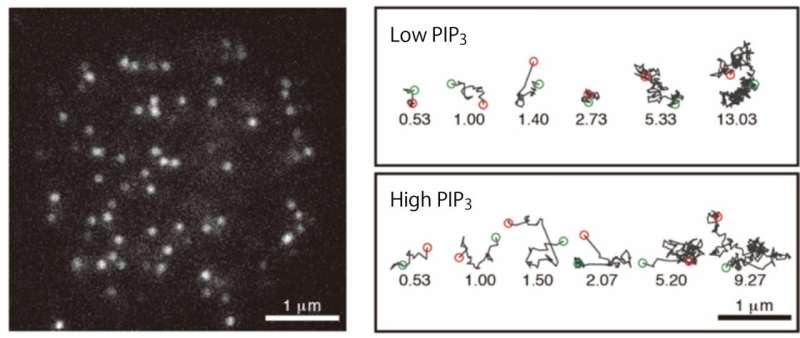
The researchers used a number of single-cell and single-molecule experimental approaches to analyze the PIP3 and PTEN molecules in slime mold and determine how their polarized distribution arises. First, they showed that when PTEN was absent from the cells, PIP3 became distributed across the whole of the cell membrane, which led to multiple pseudopodia being generated. This in turn prevented cell movement. They also quantified the levels of PIP3 and PTEN, and their specific cellular distributions, and showed that they were exclusively distributed in different regions on the membrane, with a clear boundary between them.
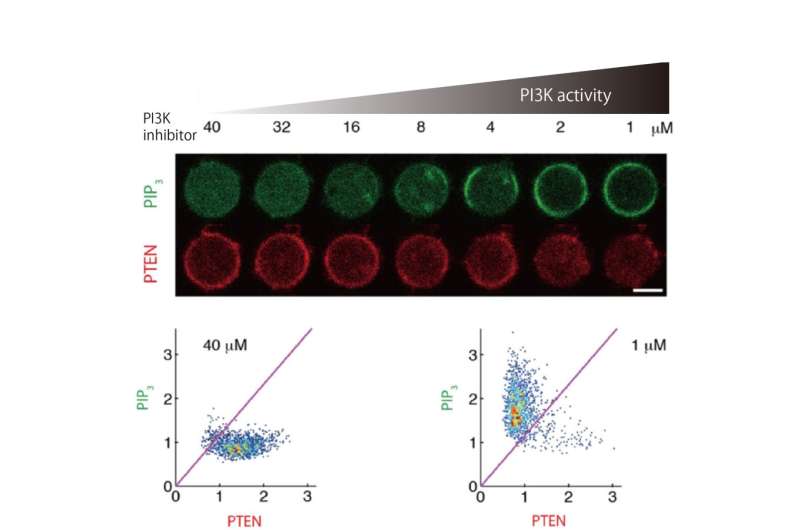
"Our findings reveal that PTEN and PIP3 function as an ultrasensitive switch in cells," says author Masahiro Ueda. "The presence of PTEN and PIP3 means they mutually suppress each other, which prevents cells from forming pseudopodia at different ends. This is an extremely effective way of guaranteeing that cells will generate propulsive forces in only one direction, avoiding wasted energy."
Given that PTEN and PIP3 appear to exert similar functions in a range of organisms from slime mold up to mammals, these findings could explain cell motility in many species. The setup of having two positive feedback loops of mutually inhibitory molecules may also function in other signaling pathways, given the efficiency with which it enables ultrasensitive switching between different states.
"Our work also hints at how this system might function to promote cell viability," says lead author Satomi Matsuoka. "For example, when a chemoattract is present with a particular concentration gradient, it causes the PIP3-rich region in the cell membrane to orient itself relative to that gradient, which in turn induces propulsion in the direction of the gradient. In this way, cells are automatically induced to move towards or away from stimuli and toxic chemicals in their environment."
More information: Satomi Matsuoka et al. Mutual inhibition between PTEN and PIP3 generates bistability for polarity in motile cells, Nature Communications (2018). DOI: 10.1038/s41467-018-06856-0
Journal information: Nature Communications
Provided by Osaka University