Organic ferromagnetism: Trapping spins in the glassy state of an organic network structure
An international team of researchers affiliated with UNIST has introduced an exciting new organic network structure that shows pure organic ferromagnetism from pure p-TCNQ without any metal contamination at room temperature. The results have been published in Chem.
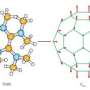
This breakthrough has been led by by Professor Jong-Beom Baek and his research team in the School of the Energy and Chemical Engineering at UNIST. In the study, the research team has synthesized a network structure from the self polymerization of tetracyanoquinodimethane (TCNQ) monomer. The designed organic network structure generates stable neutral radicals.
For over two decades, there has been widespread skepticism around claims of organic plastic ferromagnetism, mostly due to contamination by transition metals. Extensive effort has been devoted to developing magnets in purely organic compounds based on free radicals, driven by both scientific curiosity and the potential applications of a "plastic magnet." Researchers have therefore sought to exclude the contamination issues and realize magnetic properties from pure organic plastics.
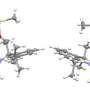
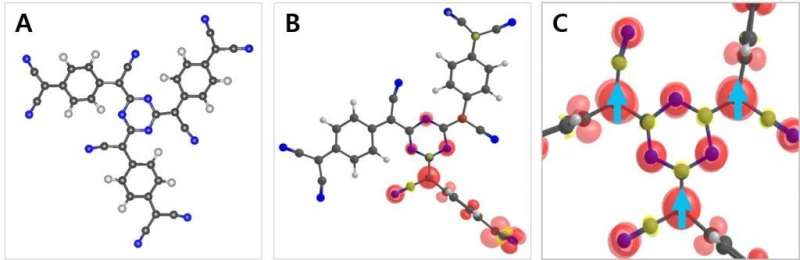
This research work reports the design, synthesis and magnetic properties of a triazine network structure demonstrating room-temperature ferromagnetism derived from pure organic material. The polymer network was realized through the self-polymerization of TCNQ in trifluoromethanesulfonic acid (TFMSA) at 155 degrees C . Highly stable free radicals are generated by twisting π bonds around the triazine rings and by trapping in the glassy state of a polymerized TCNQ (p-TCNQ) network structure.

The presence of unpaired electrons (radicals) in the p-TCNQ was confirmed by solid-state electron spin resonance (ESR) spectroscopy and magnetic characterization revealed the presence of spin ½ moments, which leads to ferromagnetic ordering with a critical temperature significantly higher than room temperature. The experimental results were supported by rigorous theoretical calculation to verify the origin of organic ferromagnetism.
This study has been jointly conducted by Javeed Mahmood of Energy and Chemical Engineering, Jungmin Park of Material Science and Engineering, and Dongbin Shin of Department of Physics at UNIST. Professor Jong-Beom Baek and Professor Jung-Woo Yoo of Material Science and Engineering supervised the project as a corresponding authors of this study.
"Our study not only suggests new directions in organic magnetic materials, but also presents a wide range of possibilities for designing new structures with neutral stable radicals, which show ferromagnetic ordering," says Professor Baek. "This material is expected to attract attention in many areas because of the scientific curiosity and the potential applications of plastic magnets."
More information: Javeed Mahmood et al. Organic Ferromagnetism: Trapping Spins in the Glassy State of an Organic Network Structure, Chem (2018). DOI: 10.1016/j.chempr.2018.07.006
Journal information: Chem