Embryonic mammary gland stem cells identified
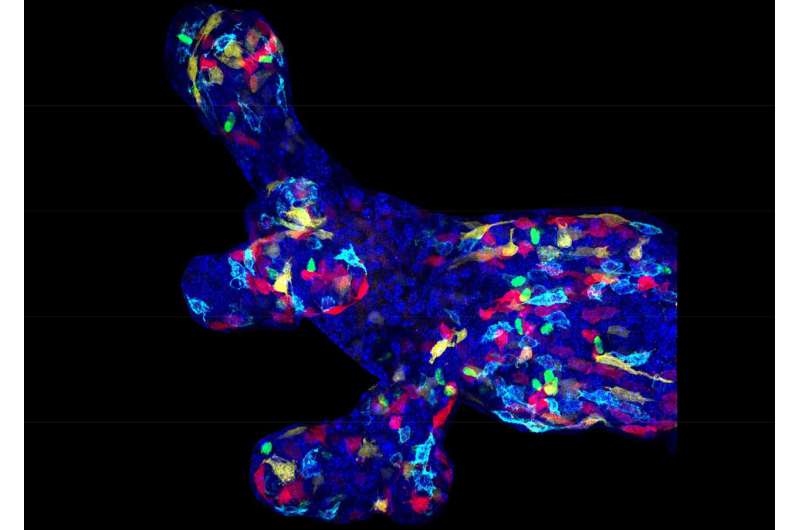
Research team led by Prof. Cédric Blanpain has identified the mechanisms that regulate mammary gland development. Using a combination of lineage tracing, molecular profiling, single cell sequencing and functional experiments, A. Wuidart and colleagues demonstrated that mammary gland development is initiated by multipotent progenitors during the early steps of embryonic mammary gland morphogenesis, whereas postnatal mammary gland development is mediated by lineage-restricted stem cells.
Their conclusions are published in Nature Cell Biology.
The mammary gland is the tissue that produces milk during lactation, allowing the survival of young mammalian offspring. The mammary gland is composed of basal cells, which surround the inner luminal cells. The luminal cells can be subdivided into ductal cells and alveolar cells that produce the milk. The basal cells allow the circulation of the milk from the alveoli to the nipple region through their contractile properties. During mammary gland expansion during puberty and into adult life, distinct pools of unipotent stem cells replenish the basal and luminal lineages independently of each other. However, it remains unclear how mammary gland tissue initially develops. Are embryonic mammary gland progenitors multipotent, meaning that their progenitors are capable of giving rise to both basal and luminal cells? If so, when does the switch from multipotency to unipotency occur? And what are the molecular mechanisms that regulate multipotency and basal and luminal lineage segregation?
The research team led by Prof. Cédric Blanpain, MD/Ph.D., professor at the Université libre de Bruxelles, Belgium, report that by using a combination of lineage tracing, molecular profiling, single cell sequencing and functional experiments, Aline Wuidart and colleagues demonstrated that the mammary gland initially develops from multipotent progenitors during the early steps of embryonic mammary gland morphogenesis, whereas postnatal mammary gland development is mediated by lineage-restricted stem cells.
To understand the molecular mechanisms regulating multipotency during embryonic development, the researchers developed a novel strategy to isolate embryonic mammary gland stem cells, and assessed for the first time their molecular features using single cell sequencing in collaboration with Thierry Voet group, KUL/Sanger Institute. Interestingly, only embryonic mammary gland progenitors, but not adult cells, expressed a hybrid transcriptional signature comprising markers for both luminal and basal lineages, explaining their multipotent fate at this stage of embryonic development.
Breast cancer is the most common cancer among women. By analyzing the early steps of breast cancer formation, Alexandra Van Keymeulen and Cédric Blanpain previously demonstrated that the expression of one of the most frequently mutated genes in patients with breast cancers reactivates a multipotent program in adult unipotent stem cells. In this new study, the researchers show that embryonic mammary gland progenitors express the same genes as during the reactivation of multipotency associated with breast cancer development.
"It was really interesting to see that many genes found to be specifically expressed by embryonic mammary gland progenitors are expressed in aggressive human breast cancers with poor prognosis, further suggesting that the reactivation of a gene expression program characteristic of embryonic mammary gland during tumorigenesis is essential for cancer growth and invasion," says Cédric Blanpain, the senior author of this study.
In conclusion, this new study identifies multipotent embryonic stem cells of the mammary gland, uncovers the molecular features associated with embryonic multipotency and identifies the molecular mechanisms regulating the switch from multipotency to unipotency during mammary gland development. The paradigm reported in this study has important implications for understanding the development of other glandular organs and tissues such as the prostate that present similar developmental switch. Finally, the mechanisms uncovered here also have implications for cancer development and progression.
More information: Aline Wuidart et al, Early lineage segregation of multipotent embryonic mammary gland progenitors, Nature Cell Biology (2018). DOI: 10.1038/s41556-018-0095-2
Journal information: Nature Cell Biology
Provided by Université libre de Bruxelles