Paucity of phosphorus hints at precarious path for extraterrestrial life

Work by Cardiff University astronomers suggests there may be a cosmic lack of a chemical element essential to life. Dr. Jane Greaves and Dr. Phil Cigan will present their results at the European Week of Astronomy and Space Science in Liverpool.
Greaves has been searching for phosphorus in the universe, because of its link to life on Earth. If this element—with the chemical code P—is lacking in other parts of the cosmos, then it could be difficult for extra-terrestrial life to exist.
She explains: "Phosphorus is one of just six chemical elements on which Earth organisms depend, and it is crucial to the compound adenosine triphosphate (ATP), which cells use to store and transfer energy. Astronomers have just started to pay attention to the cosmic origins of phosphorus and found quite a few surprises. In particular, P is created in supernovae—the explosions of massive stars—but the amounts seen so far don't match our computer models. I wondered what the implications were for life on other planets if unpredictable amounts of P are spat out into space and later used in the construction of new planets."
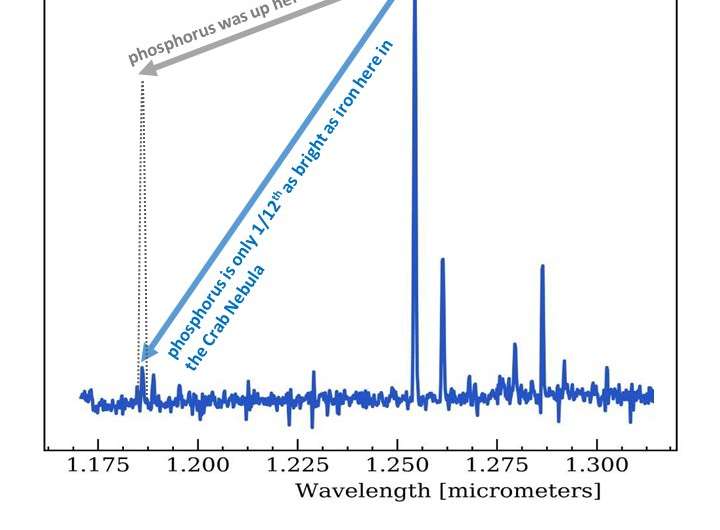
The team used the UK's William Herschel Telescope, sited on La Palma in the Canary islands, to observe infrared light from phosphorus and iron in the Crab Nebula, a supernova remnant around 6500 light years away in the direction of the constellation of Taurus.

Cigan, an expert on these stellar remnants, says: "This is only the second such study of phosphorus that has been made. The first looked at the Cassiopeia A (Cas A) supernova remnant, and so we are able to compare two different stellar explosions and see if they ejected different proportions of phosphorus and iron. The first element supports life, while the second is a major part of our planet's core".
The astronomers struggled with foggy nights at the telescope, back in November 2017, and are only just starting to get scientific results from a few hours of data.
Cigan cautions "These are our preliminary results, which we extracted only in the last couple of weeks! But at least for the parts of the Crab Nebula we were able to observe so far, there seems to be much less phosphorus than in Cas A. The two explosions seem to differ from each other, perhaps because Cas A results from the explosion of a rare super-massive star. We've just asked for more telescope time to go back and check, in case we've missed some phosphorus-rich regions in the Crab Nebula."
The preliminary results suggest that material blown out into space could vary dramatically in chemical composition. Greaves remarks: "The route to carrying phosphorus into new-born planets looks rather precarious. We already think that only a few phosphorus-bearing minerals that came to the Earth—probably in meteorites—were reactive enough to get involved in making proto-biomolecules.
'If phosphorus is sourced from supernovae, and then travels across space in meteoritic rocks, I'm wondering if a young planet could find itself lacking in reactive phosphorus because of where it was born? That is, it started off near the wrong kind of supernova? In that case, life might really struggle to get started out of phosphorus-poor chemistry, on another world otherwise similar to our own."
The researchers now plan to continue their search, to establish whether other supernova remnants also lack phosphorus, and whether this element, so important for complex life, is rarer than we thought.
Provided by Royal Astronomical Society