Energy level alignment for molecular electronics
NUS physicists have found that complex electron-electron interactions change the energy levels at molecule-metal interfaces, affecting the performance of molecular electronic devices.
Molecular electronics involves the use of molecules as the main building block for creating the electronic circuitry. It can potentially be used to develop circuits that are much smaller than those made from conventional silicon processes. Understanding the electronic properties of the interface between the molecules and metal conductors, particularly their associated energy levels, is important for rationalising and optimising device performance. This is central to the development of molecular electronics.
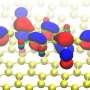
A fundamental property of every molecule is its energy gap, defined as the energy difference between the highest and lowest orbital energy level occupied and unoccupied by electrons respectively. These levels are also the most important energy levels for device performance. The energy gap of a molecule becomes smaller when the molecule is brought close to a metal surface; this will make it easier for charge carriers to move between the molecule and the metal contact. This change in gap is primarily caused by electronic screening effects from the metal surface, and can be as large as several electron-volts. However, this electronic screening effect is missing from the majority of theoretical studies on this topic.
A research team led by Prof Su Ying QUEK, from the Department of Physics, NUS has elucidated the interface electronic structure properties for a number of different molecules on gold surfaces using state-of-the-art theoretical and computational methods that explicitly take into account electronic screening effects from first principles. The researchers carried out computations on molecular systems anchored by common chemical functional groups (amine, pyridine and thiolate groups). The research team found that for a single molecule, the electronic screening effect can be accurately predicted from an image charge model, even in the presence of chemical bonds. The image charge model is a classical electrostatics method which approximates the electronic screening of a test charge by an image charge in the metal. However, in devices with many molecules, the researchers found significant additional electronic screening mechanisms. Besides intermolecular screening effects, substrate-mediated intermolecular interactions are also found to contribute to these additional screening mechanisms. The findings suggest that charge carriers can tunnel more easily across the interface in devices with many molecules.
Prof Quek said, "This work provides valuable insights into the many electron effects at the molecule-metal interfaces involving chemical bonds. The results and findings from this research constitute an important step towards the understanding and manipulation of functional organic systems in the development of molecular devices."
More information: Yifeng Chen et al. Energy Level Alignment at Hybridized Organic–Metal Interfaces: The Role of Many-Electron Effects, The Journal of Physical Chemistry C (2017). DOI: 10.1021/acs.jpcc.7b00715
Journal information: Journal of Physical Chemistry C
Provided by National University of Singapore