Interactions between simple molecular mechanisms give rise to complex infection dynamics

Bacteria, which cause infections, can themselves be infected by viruses called bacteriophages. Just as not all bacteria are harmful to humans, not all viruses are harmful to bacteria, and some can even benefit them. Can bacteria distinguish good and bad viruses? An interdisciplinary team of scientists at the Institute of Science and Technology Austria (IST Austria) studied how infections with potentially beneficial viruses play out in bacteria that carry a certain type of anti-viral immune mechanism called restriction-modification (RM). They show that population-level interactions between viruses and bacteria make the infection proceed in a way that compensates for the inherent disadvantage of individual cells and allows immune bacteria to acquire many more beneficial viruses in the long term. This is the finding of a study published in Nature Ecology & Evolution. The study was carried out by Maros Pleska, a PhD student and Moritz Lang a postdoc in the group of Celin Guet at IST Austria, as well as collaborators Dominik Refardt at Zurich University of Applied Sciences and Bruce Levin at Emory University.
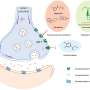
Many viruses simply replicate inside bacteria, and such infections, which usually result in the death of the infected bacterium, are called lytic. However, some viruses, called temperate viruses, can take a gentler approach: During lysogenic infections, the genetic information of a temperate virus integrates into the genetic information of the infected bacterium and thus enhances the bacterial gene repertoire. Examples of genes, which are spread among bacteria by temperate viruses, include dangerous toxins such as shiga toxin, cholera toxin, or botulinum toxin. However, there is a catch for the bacteria, as temperate viruses can both kill or integrate into their hosts, and the decision on which way the infection proceeds is made in a seemingly random manner.
It is well known that many bacteria protect themselves from lethal lytic infections by carrying mechanisms of immunity, such as restriction-modification (RM) systems that cut viral DNA. What is not known is how these primitive immune systems affect the ability of bacteria to acquire potentially beneficial viruses in the process of lysogeny. Can RM systems distinguish between lytic and lysogenic infections, or do they act more generally? And while protecting bacteria from death, do they also prevent them from acquisition of beneficial viruses? Pleska, Lang and their colleagues combined experiments and theory to investigate this question.
As a first step, the researchers looked at what happens to individual bacteria carrying RM systems when infected with temperate viruses. They found that the RM system always sought to prevent infection, regardless of whether the infection was heading towards lysis or lysogeny. From this, the conclusion is that, as an inadvertent cost of preventing bacteria from lysis, RM systems are also a barrier to acquisition of viral genes.
However, a very different result emerged when the researchers investigated what happens at the level of the bacterial population. They mixed a large number of bacteria with a large number of bacteriophages, and looked at how many bacteria acquired viral genes in the long term. Based on the previous result, the scientists expected that viruses would integrate much less often in bacteria with RM systems, as compared to bacteria without this mechanism of immunity. However, the opposite happened—more viruses had integrated into bacteria that were expected to be immune.
Restriction-modification systems offer temporary respite
What is the explanation of this counterintuitive result? The researchers found that instead of preventing infections completely, RM systems merely postpone the infection, giving the bacterial population time to grow until viruses break through the barrier and infection fully hits. While lysis is more frequent in small bacterial populations and many bacteria get killed if the population is infected early on, lysogeny becomes more prevalent in large bacterial populations, making bacteria that get infected later more likely to acquire the virus instead of getting killed by it. RM systems therefore offer a temporary respite, protecting bacterial populations only from the most dangerous phase of infection, without limiting the potential benefits.
Is a new unknown molecular mechanism responsible for this switch in behaviour? No, says first author Maros Pleska: "The most intriguing finding is that we actually did not have to find any new mechanism at all. What we observe is all a simple consequence of the population dynamics of interactions between bacteria and viruses."
This result is a rebuff to those seeking to predict the longer-term behaviour of populations from the biology of individual molecular components, explains Pleska: "The basic biology of all the elements in our system was known as the RM systems and viruses we looked at are some of the best understood molecular systems we know. Nevertheless, this study illustrates how hopeless we are when it comes to using this molecular-level knowledge to predict the dynamics that occur after we put individual pieces together. In fact, our observations ran completely opposite to what anyone would expect. Thus, ecological and evolutionary interactions between even the simplest biological elements can be very complex and we need new ways of looking at them, if we ever want to understand their role in nature."
More information: Maroš Pleška et al, Phage–host population dynamics promotes prophage acquisition in bacteria with innate immunity, Nature Ecology & Evolution (2017). DOI: 10.1038/s41559-017-0424-z
Journal information: Nature Ecology & Evolution
Provided by Institute of Science and Technology Austria